Using The Activity Series Provided Which Reactants Will Form Products
Using The Activity Series Provided Which Reactants Will Form Products - Web based on the activity series provided, which reactants will form products? Particles of the products will be present, but no particles of the reactants will remain. Among these processes which is the slowest chemical reaction. Using the activity series provided. Web based on the activity series provided, which reactants will form products? Na > mg > al > mn > zn > cr > fe > cd >. Which reactants will form products? Which reactants will form products? Web using the activity series provided. Which reactants will form products?
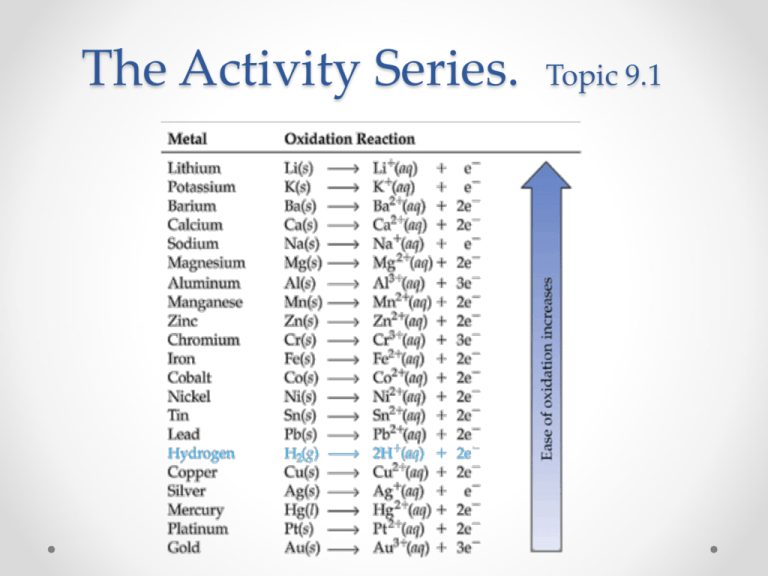
Na > mg > al > mn > zn > cr > fe > cd >. Web which reactants will form products. Na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h > sb > bi > cu > ag 1. Web both reactants and products will be present, but forward and reverse reactions will stop. | wyzant ask an expert chemistry conversion curry f. F > cl > br > i cui2 + br2 right arrow. Particles of the products will be present, but no particles of the reactants will remain. Web using the activity series provided. Web using the activity series provided. Na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h > sb > bi > cu > ag ag +nano3 → fe +.
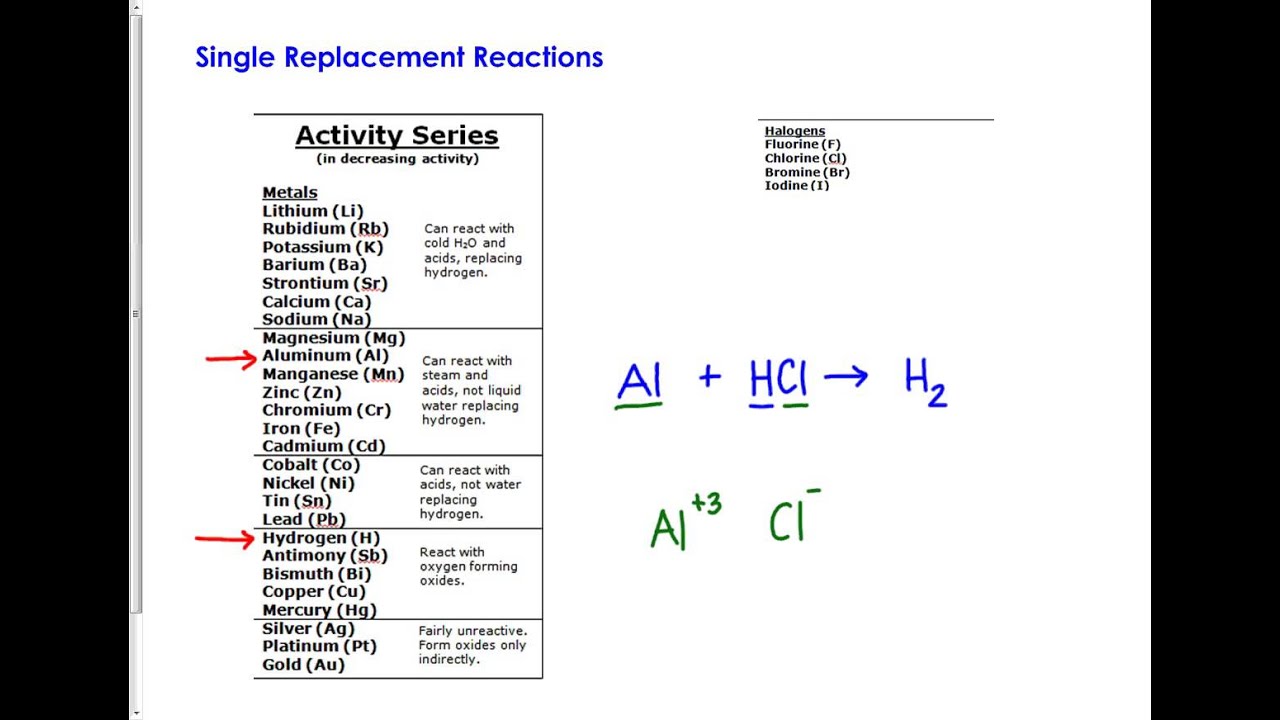
Al &gt… get the answers you need, now! Web based on the activity series provided, which reactants will form products? Web which reactants will form products. Among these processes which is the slowest chemical reaction. Na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h > sb > bi > cu > ag ag +nano3 → fe +. Na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h > sb > bi > cu > ag 1. | wyzant ask an expert chemistry conversion curry f. Web (quiz question) using the activity series provided. Web using the activity series, the products of the reaction will be copper (cu) and iron(ii) nitrate (fe(no₃)₂. Which of the following is.
Redox Reactions Part B Activity Series Boulance Products Observations
Web in a chemical reaction, reactants contact each other, bonds between atoms in the reactants are broken, and atoms rearrange and form new bonds to brand the. Web (quiz question) using the activity series provided. Web while looking at the reactants only, whether a reaction will occur or not can be determined by comparing the location of the metals within.
What Is a Reversible Reaction?
Web while looking at the reactants only, whether a reaction will occur or not can be determined by comparing the location of the metals within the reactants on an. Web which reactants will form products?na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h >.
Topic 9.1 Activity Series
Web (quiz question) using the activity series provided. Which reactants will form products? A more reactive species such as f 2 can. Using the activity series provided. Na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h > sb > bi > cu > ag.
Cellular Energy Worksheet
Web which reactants will form products?na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h > sb > bi > cu > ag fe + cu (no3)2 considering the activity series given. Al &gt… get the answers you need, now! Which reactants will form products?.
What Is a Chemical Reaction? — Overview & Examples Expii
Using the activity series provided. Which reactants will form products? Web based on the activity series provided, which reactants will form products? Which reactants will form products? Web using the activity series provided.
How to Use the Activity Series YouTube
Web 06/21/2022 chemistry high school answered using the activity series provided. Web while looking at the reactants only, whether a reaction will occur or not can be determined by comparing the location of the metals within the reactants on an. Web using the activity series, the products of the reaction will be copper (cu) and iron(ii) nitrate (fe(no₃)₂. Web using.
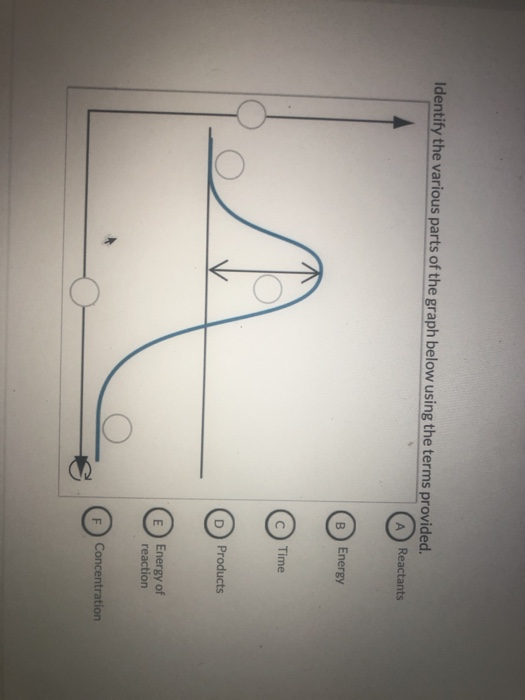
Solved Identify the various parts of the graph below using
Which reactants will form products? A more reactive species such as f 2 can. Web which reactants will form products?na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h > sb > bi > cu > ag fe + cu (no3)2 considering the activity series.
The Activity Series YouTube
Web based on the activity series provided, which reactants will form products? Web using the activity series provided. Which reactants will form products? Web which reactants will form products?na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h > sb > bi > cu >.
Chemical Reactions and Chemical Equations Owlcation
| wyzant ask an expert chemistry conversion curry f. Web (quiz question) using the activity series provided. Web using the activity series provided. Web using the activity series provided which reactants will form products | all the reactions given are single displacement reactions and can only occur if the metal is more reactive. Na > mg > al > mn.
| Wyzant Ask An Expert Chemistry Conversion Curry F.
Web which reactants will form products?na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h > sb > bi > cu > ag fe + cu (no3)2 considering the activity series given. Which reactants will form products? Which reactants will form products? Web using the activity series, the products of the reaction will be copper (cu) and iron(ii) nitrate (fe(no₃)₂.
Among These Processes Which Is The Slowest Chemical Reaction.
Web using the activity series provided. Which reactants will form products? Web while looking at the reactants only, whether a reaction will occur or not can be determined by comparing the location of the metals within the reactants on an. Which reactants will form products?
Web Using The Activity Series Provided.
Na > mg > al > mn > zn > cr > fe > cd > co > ni > sn > pb > h > sb > bi > cu > ag 1. Web which reactants will form products. Web based on the activity series provided, which reactants will form products? Web based on the activity series provided, which reactants will form products?
Web Based On The Activity Series Provided, Which Reactants Will Form Products?
Web using the activity series provided which reactants will form products | all the reactions given are single displacement reactions and can only occur if the metal is more reactive. Na > mg > al > mn > zn > cr > fe > cd >. Al &gt… get the answers you need, now! Web using the activity series provided.

/simple-experiment-58b5b3325f9b586046bbfa7f.jpg)