What Ion Does Chlorine Form
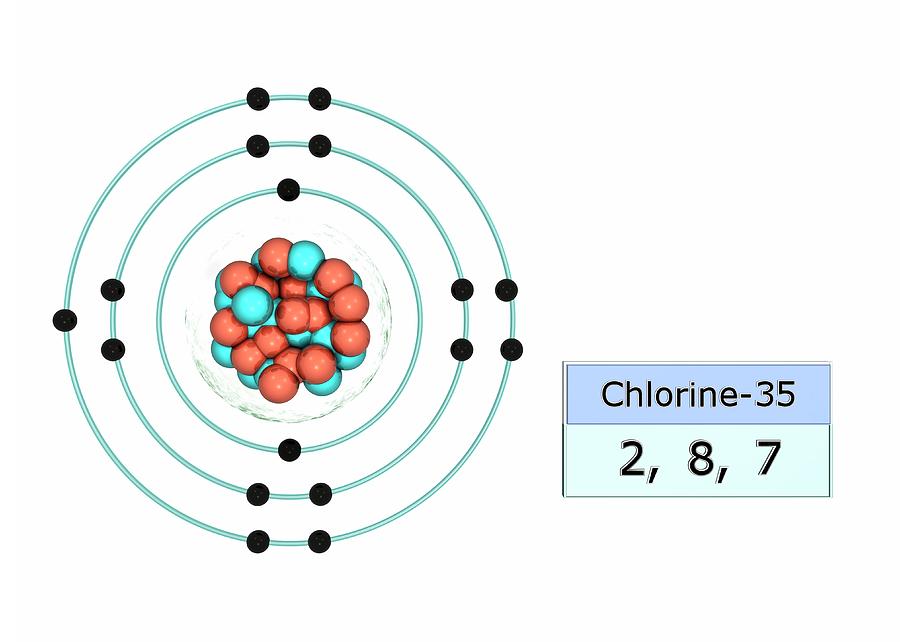
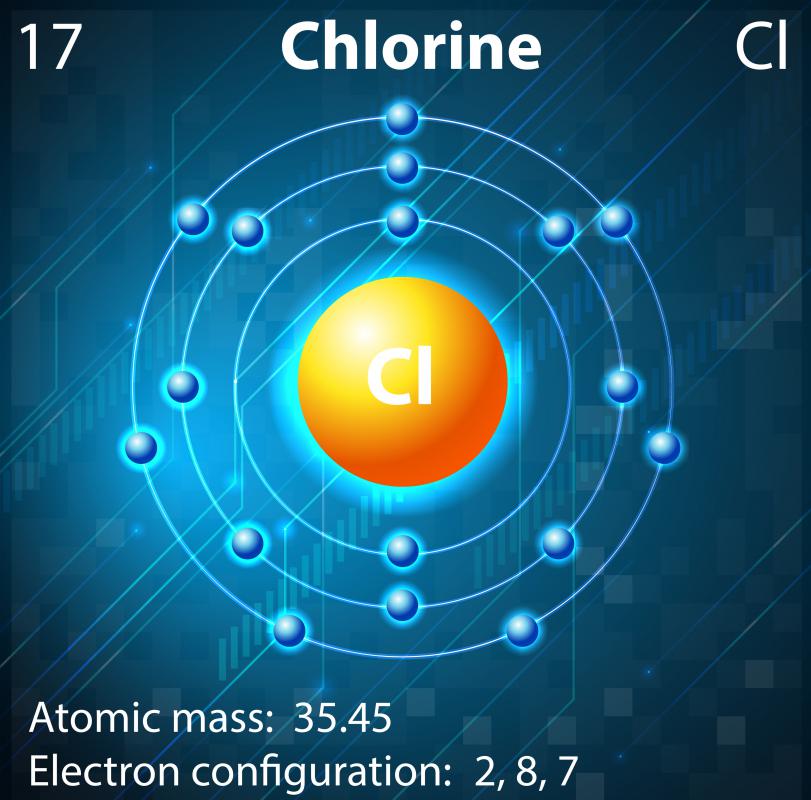
What Ion Does Chlorine Form - Chlorine is the second lightest halogen and is represented as cl. Thus, the electronic configuration of the chlorine atom is (2, 8, 7). Web aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states, respectively, in the following ions: Chemical reactions reactions with water chlorine is only. It is a member of the halogen group of elements,. Since it has 1 more electron than protons, chlorine has a charge of 1, making it a negative ion. Chlorine is a chemical element with atomic number 17 and element symbol cl. Web what ions can chlorine form? The atomic number of this. The chloride ion /ˈklɔːraɪd/ is the anion (negatively charged ion) cl−.
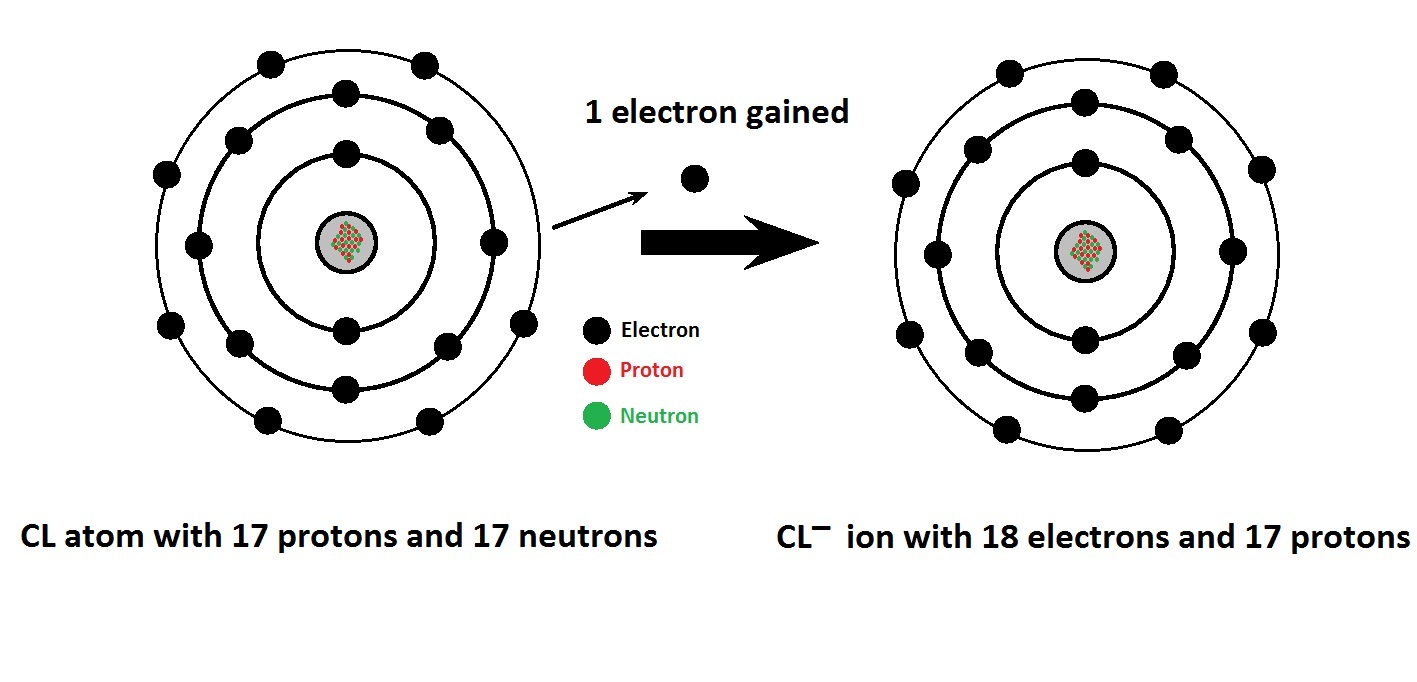
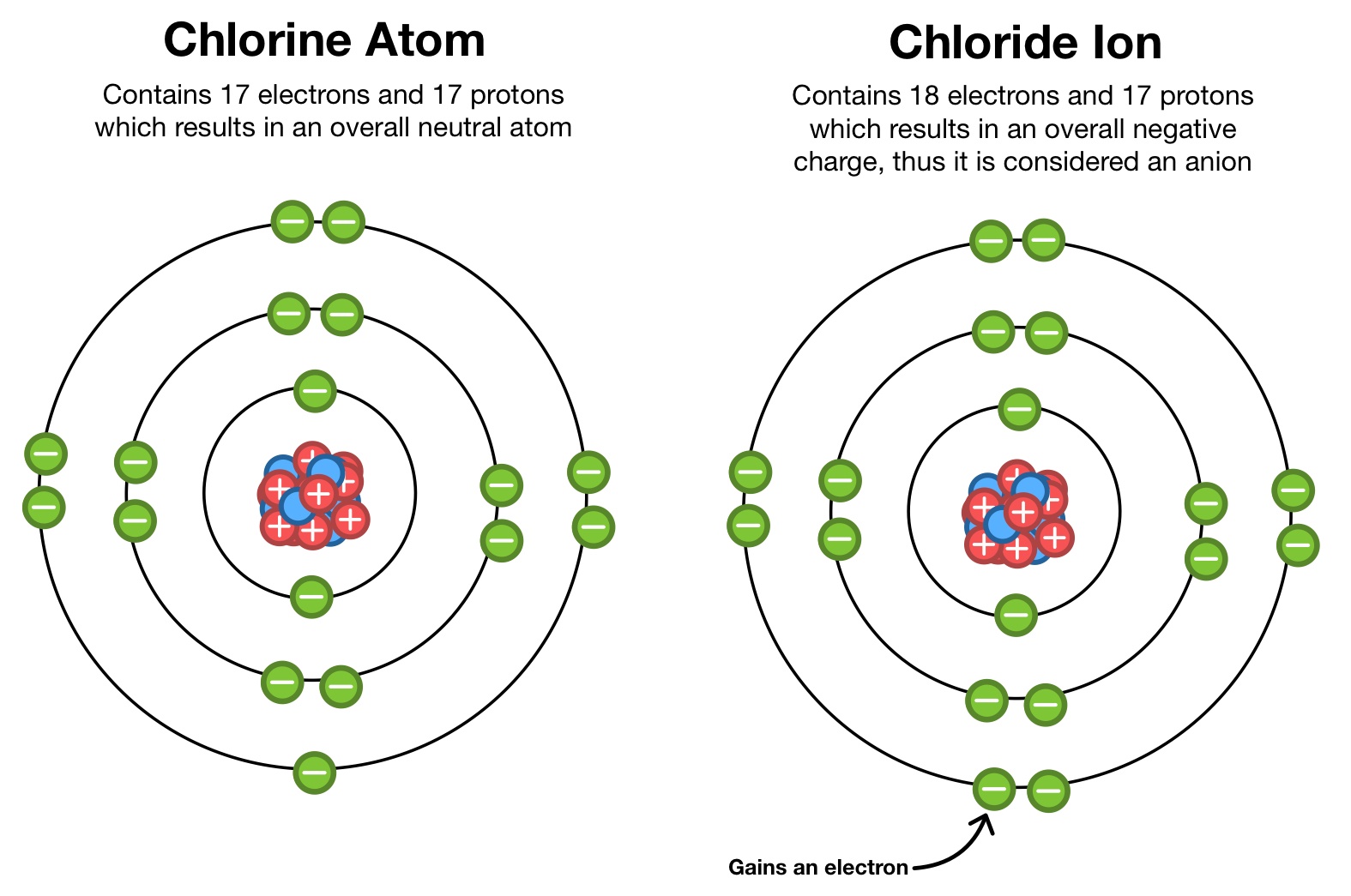
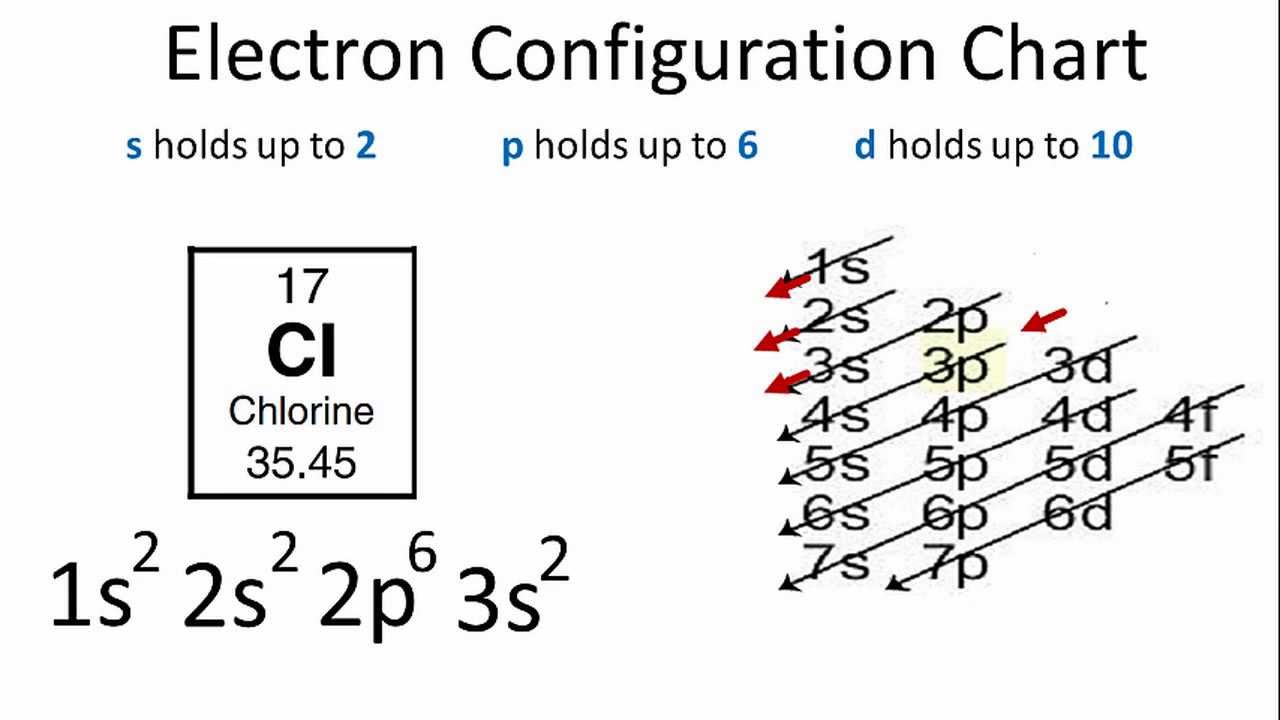
It is formed when the element chlorine (a halogen) gains an. Chlorine is the second lightest halogen and is represented as cl. Hypochlorite (clo −), chlorite (clo −. Thus, the electronic configuration of the chlorine atom is (2, 8, 7). Web aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states, respectively, in the following ions: Web what ions can chlorine form? Web chlorine is is in atomic number 17 in periodic table. The electron configuration of chlorine is 1s2 2s2 2p6 3s2 3p5 or[ne]3s2 3p5 or 2.8.7. Web chlorine gains an electron, leaving it with 17 protons and 18 electrons. Web formation of chloride ion from chlorine atom:
Web chlorine chemistry is perhaps best known for its role in swimming pools, drinking water, and household surface disinfection with chlorine bleach. The electron configuration of chlorine is 1s2 2s2 2p6 3s2 3p5 or[ne]3s2 3p5 or 2.8.7. This fills the chlorine atom's outer shell, making it electronically stable. Web aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states, respectively, in the following ions: Web what ions can chlorine form? The chloride ion /ˈklɔːraɪd/ is the anion (negatively charged ion) cl−. Web chlorine is is in atomic number 17 in periodic table. Web updated on november 07, 2019. Web formation of chloride ion from chlorine atom: Chemical reactions reactions with water chlorine is only.
Chlorine Cl (Element 17) of Periodic Table Newton Desk
Web chlorine gains an electron, leaving it with 17 protons and 18 electrons. However, note that chlorine can also occur in polyatomic ions, especially. Thus, the electronic configuration of the chlorine atom is (2, 8, 7). Chlorine atom (cl) has atomic number 17. The electron configuration of chlorine is 1s2 2s2 2p6 3s2 3p5 or[ne]3s2 3p5 or 2.8.7.
Basic Chemistry October 2012
Thus, the electronic configuration of the chlorine atom is (2, 8, 7). Chlorine is the second lightest halogen and is represented as cl. Web chlorine is is in atomic number 17 in periodic table. Since it has 1 more electron than protons, chlorine has a charge of 1, making it a negative ion. Web what ions can chlorine form?
Draw the atomic structure of a chlorine ion Brainly.in
Chlorine ions are presumably attacking the passivation layer on the surface of magnesium alloys and can therefore enhance the aggressiveness of the saline solution. However, note that chlorine can also occur in polyatomic ions, especially. Since it has 1 more electron than protons, chlorine has a charge of 1, making it a negative ion. Chemical reactions reactions with water chlorine.
Ions — Definition & Overview Expii 7BE
It is a member of the halogen group of elements,. Since it has 1 more electron than protons, chlorine has a charge of 1, making it a negative ion. However, note that chlorine can also occur in polyatomic ions, especially. Chlorine is a chemical element with atomic number 17 and element symbol cl. Chemical reactions reactions with water chlorine is.
Chlorine Facts, Symbol, Discovery, Properties, Uses
The atomic number of this. Web xik jun 21, 2016 a chloride ion forms when a chlorine atom gains an electron. Chlorine is a chemical element with atomic number 17 and element symbol cl. It is formed when the element chlorine (a halogen) gains an. Web aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5,.
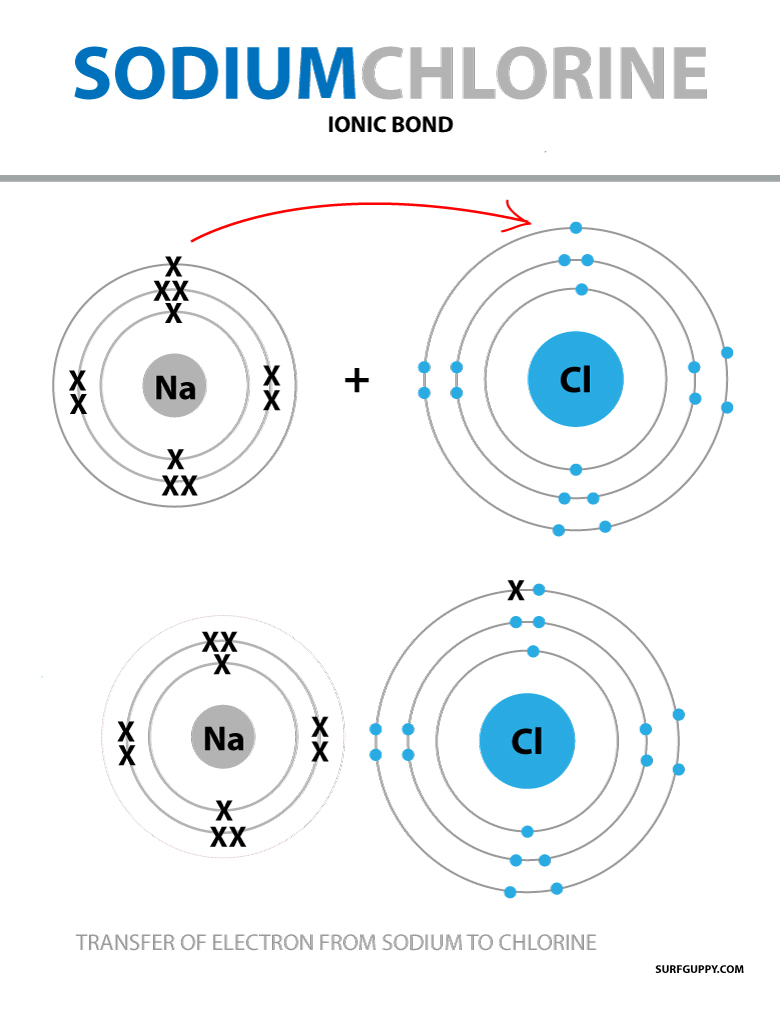
What is Ionic Bond Surfguppy Chemistry made easy visual learning
Web chlorine gains an electron, leaving it with 17 protons and 18 electrons. Web aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states, respectively, in the following ions: Thus, the electronic configuration of the chlorine atom is (2, 8, 7). Since it has 1 more electron than protons, chlorine has a.
The Electron 2020 Give The Electron Configuration Of Chlorine
However, note that chlorine can also occur in polyatomic ions, especially. The electron configuration of chlorine is 1s2 2s2 2p6 3s2 3p5 or[ne]3s2 3p5 or 2.8.7. Hypochlorite (clo −), chlorite (clo −. The atomic number of this. Chlorine atom (cl) has atomic number 17.
How To Find The Electron Configuration For Chlorine Dynamic Periodic
The electron configuration of chlorine is 1s2 2s2 2p6 3s2 3p5 or[ne]3s2 3p5 or 2.8.7. Chemical reactions reactions with water chlorine is only. Web chlorine chemistry is perhaps best known for its role in swimming pools, drinking water, and household surface disinfection with chlorine bleach. But did you know that chlorine. Web what ions can chlorine form?
Chlorine Electron Configuration Photograph by
Web chlorine gains an electron, leaving it with 17 protons and 18 electrons. Web chlorine is is in atomic number 17 in periodic table. The chloride ion /ˈklɔːraɪd/ is the anion (negatively charged ion) cl−. The atomic number of this. This fills the chlorine atom's outer shell, making it electronically stable.
What are Chlorinated Hydrocarbons? (with pictures)
This fills the chlorine atom's outer shell, making it electronically stable. Web formation of chloride ion from chlorine atom: Thus, the electronic configuration of the chlorine atom is (2, 8, 7). Web xik jun 21, 2016 a chloride ion forms when a chlorine atom gains an electron. The electron configuration of chlorine is 1s2 2s2 2p6 3s2 3p5 or[ne]3s2 3p5.
This Fills The Chlorine Atom's Outer Shell, Making It Electronically Stable.
But did you know that chlorine. The atomic number of this. Chlorine ions are presumably attacking the passivation layer on the surface of magnesium alloys and can therefore enhance the aggressiveness of the saline solution. Chlorine is a chemical element with atomic number 17 and element symbol cl.
However, Note That Chlorine Can Also Occur In Polyatomic Ions, Especially.
It is a member of the halogen group of elements,. Web updated on november 07, 2019. Chlorine atom (cl) has atomic number 17. It is formed when the element chlorine (a halogen) gains an.
Chemical Reactions Reactions With Water Chlorine Is Only.
Web chlorine gains an electron, leaving it with 17 protons and 18 electrons. Web what ions can chlorine form? Web aside from the −1 oxidation states of some chlorides, chlorine exhibits +1, +3, +5, and +7 oxidation states, respectively, in the following ions: The electron configuration of chlorine is 1s2 2s2 2p6 3s2 3p5 or[ne]3s2 3p5 or 2.8.7.
Hypochlorite (Clo −), Chlorite (Clo −.
Web chlorine is is in atomic number 17 in periodic table. The chloride ion /ˈklɔːraɪd/ is the anion (negatively charged ion) cl−. Web xik jun 21, 2016 a chloride ion forms when a chlorine atom gains an electron. Web formation of chloride ion from chlorine atom: