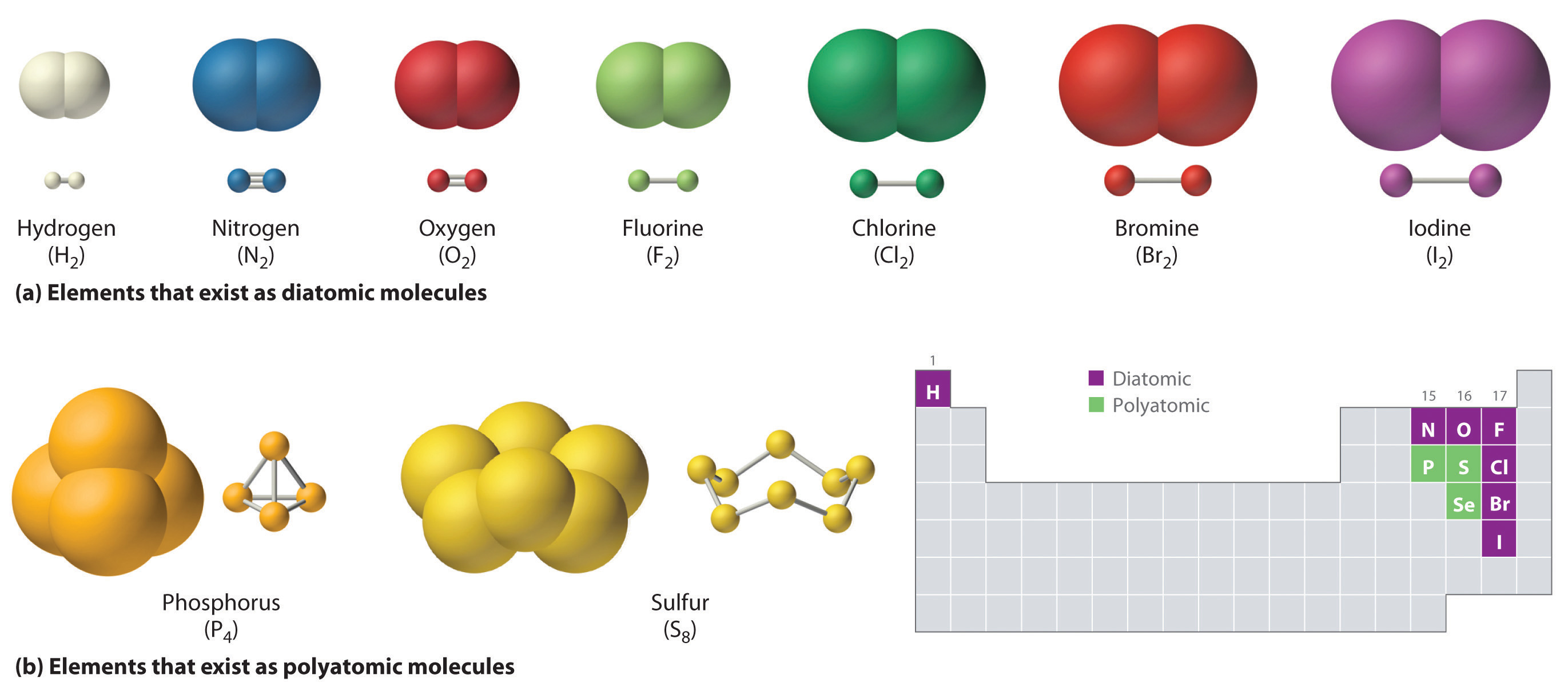
What Kinds Of Elements Form Ionic Bonds
What Kinds Of Elements Form Ionic Bonds - Web answer (1 of 3): The alkali halides (nacl, lif, etc.). An atom of sodium will lose an electron and form a positive ion. Web it is now called a sodium ion. An atom of chlorine will gain. Web what type of elements form ionic bonds? Charged chemical species form when neutral atoms, or groups of atoms, lose. 2) another example, magnesium and oxygen. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another.
Web ionic bonds are one of the two main types of chemical bonds. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Web which elements form ionic bonds? 2) another example, magnesium and oxygen. They form as a result of electrostatic attraction between oppositely charged ions and usually occur. The alkali halides (nacl, lif, etc.). The chlorine atom has seven electrons in its outer shell. Web it is now called a sodium ion. Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion. There are primarily two forms of bonding that an atom can participate in:
Web ionic bonds are one of the two main types of chemical bonds. This exchange results in a more stable, noble gas. 4/28/2022 wiki user ∙ 14y ago study now see answers (3) best answer copy metals form ionic bonds with. Web ionic and covalent bonding. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Charged chemical species form when neutral atoms, or groups of atoms, lose. The chlorine atom has seven electrons in its outer shell. 2) another example, magnesium and oxygen. Electrical conductivity how are ionic bonds formed? Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals).
Ionic Bond Definition Easy Hard Science
Web which elements form ionic bonds? An atom of sodium will lose an electron and form a positive ion. Electrical conductivity how are ionic bonds formed? Boiling and melting point 4. Web answer (1 of 3):
Examples of Ionic Bonding YouTube
They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Charged chemical species form when neutral atoms, or groups of atoms, lose. The alkali halides (nacl, lif, etc.). Web ionic bonds are one of the two main types of chemical bonds. Electrical conductivity how are ionic bonds formed?
Examples of Ionic Bonds and Compounds
2) another example, magnesium and oxygen. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Web what type of elements form ionic bonds? They form as a result of electrostatic attraction between oppositely charged ions and usually occur. 1) for example, consider na and cl.
10 Notable Differences Between Ionic And Covalent Bonds Current
Web it is now called a sodium ion. Charged chemical species form when neutral atoms, or groups of atoms, lose. 4/28/2022 wiki user ∙ 14y ago study now see answers (3) best answer copy metals form ionic bonds with. An atom of chlorine will gain. They form as a result of electrostatic attraction between oppositely charged ions and usually occur.
2.6 Molecules and Molecular Compounds Chemistry LibreTexts
The chlorine atom has seven electrons in its outer shell. Web ionic and covalent bonding. Web which elements form ionic bonds? This exchange results in a more stable, noble gas. Charged chemical species form when neutral atoms, or groups of atoms, lose.
Naming Simple Ionic Compounds Pathways to Chemistry
Boiling and melting point 4. 2) another example, magnesium and oxygen. This exchange results in a more stable, noble gas. Web which elements form ionic bonds? Web it is now called a sodium ion.
Periodic Table of Elements & Ionic Bonding Mrs. Zeringue's 7th Grade
1) for example, consider na and cl. Covalent bonding involves the sharing of electrons. Ionic bonds form when a nonmetal and a metal exchange electrons, while covalent bonds form when electrons are shared between two nonmetals. Web it is now called a sodium ion. Web answer (1 of 3):
Ionic Bond Definition, Types, Properties & Examples
The alkali halides (nacl, lif, etc.). They form as a result of electrostatic attraction between oppositely charged ions and usually occur. The chlorine atom has seven electrons in its outer shell. An atom of chlorine will gain. Boiling and melting point 4.
Ionic Properties
There are primarily two forms of bonding that an atom can participate in: Web it is now called a sodium ion. Covalent bonding involves the sharing of electrons. 2) another example, magnesium and oxygen. 1) for example, consider na and cl.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
2) another example, magnesium and oxygen. 4/28/2022 wiki user ∙ 14y ago study now see answers (3) best answer copy metals form ionic bonds with. There are primarily two forms of bonding that an atom can participate in: Web ionic and covalent bonding. 3) last example, mg and cl.
Web Ionic Bonding Occurs In Compounds Composed Of Strongly Electropositive Elements (Metals) And Strongly Electronegative Elements (Nonmetals).
Web it is now called a sodium ion. Web what type of elements form ionic bonds? Electrical conductivity how are ionic bonds formed? Web an ionic bond is a bond between two oppositively charged chemical species, a cation and an anion.
There Are Primarily Two Forms Of Bonding That An Atom Can Participate In:
An atom of chlorine will gain. The alkali halides (nacl, lif, etc.). Web ionic and covalent bonding. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another.
The Chlorine Atom Has Seven Electrons In Its Outer Shell.
1) for example, consider na and cl. 4/28/2022 wiki user ∙ 14y ago study now see answers (3) best answer copy metals form ionic bonds with. They form as a result of electrostatic attraction between oppositely charged ions and usually occur. 3) last example, mg and cl.
Boiling And Melting Point 4.
Covalent bonding involves the sharing of electrons. Web answer (1 of 3): An atom of sodium will lose an electron and form a positive ion. This exchange results in a more stable, noble gas.


/ionic-bond-58fd4ea73df78ca1590682ad.jpg)