What Types Of Atoms Form Covalent Bonds
What Types Of Atoms Form Covalent Bonds - Any object (such as a magnet, polar molecule or antenna), that is oppositely charged at two points (or poles). The sharing of bonding pairs will ensure that the atoms achieve stability in their outer shell, similar. The pair of electrons involved in this type of bonding is known as a shared pair or bonding pair. Each h atom starts with a single electron in its valence shell: Two different atoms can also share electrons and form covalent bonds. Starting on the far right, we have two separate hydrogen atoms with a particular potential energy, indicated. Web double bonds triple bond. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Ions with opposite electrical charges attract d. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent bond.
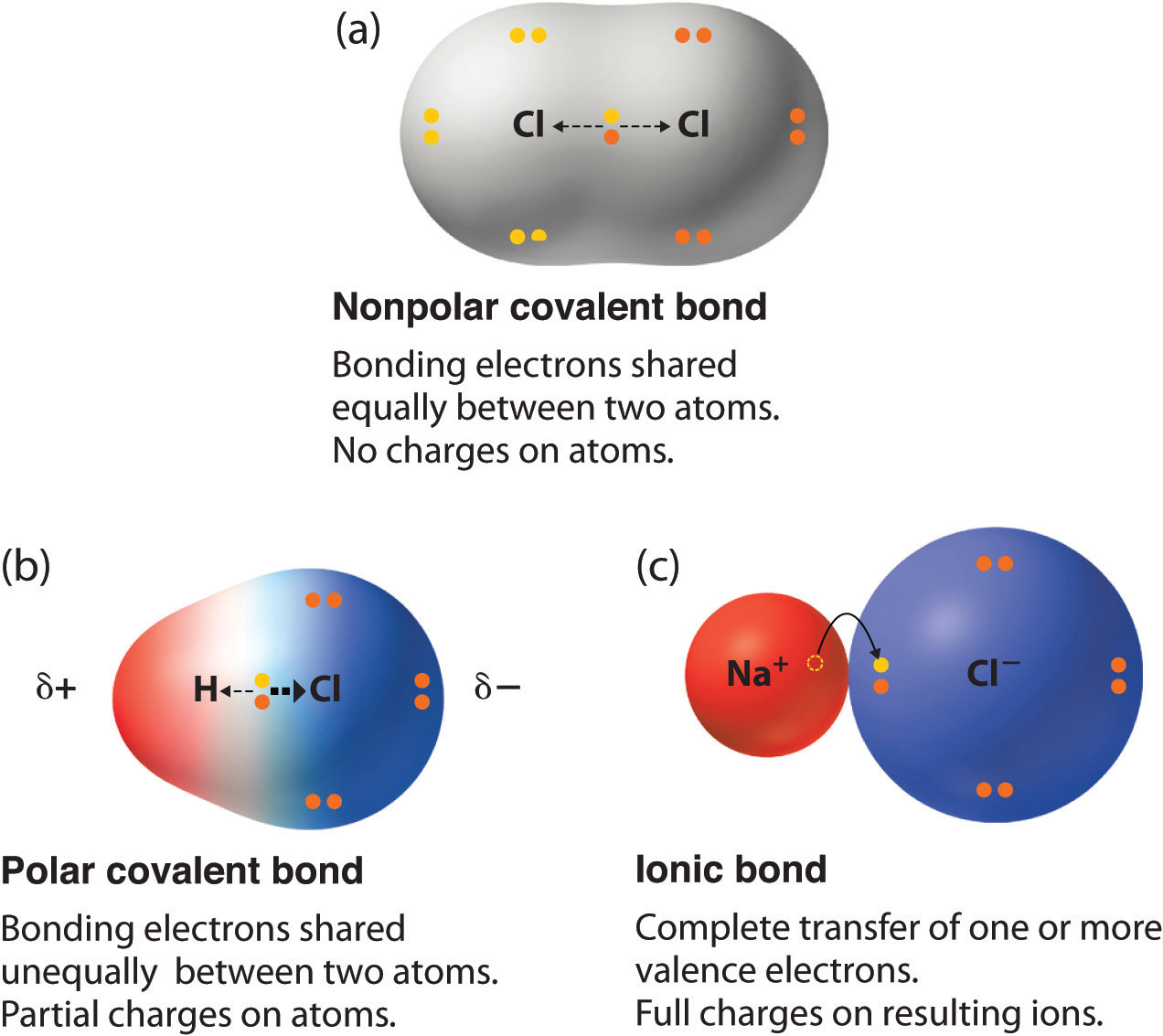
Hydrogen bonds and london dispersion forces. In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. In general, bonds are considered to be covalent if the electronegativity difference between the two atoms bonding is less than 2.0 pauling units. Web a covalent bond is formed when electrons from both participating atoms are shared equally. The pair of electrons involved in this type of bonding is known as a shared pair or bonding pair. Web there are two main types of covalent bonds that can occur based on the electronegativity of the atoms involved: This type of covalent bond is. Starting on the far right, we have two separate hydrogen atoms with a particular potential energy, indicated by the red line. Various methods of showing a covalent bond. The simplest covalent bond exists in the diatomic hydrogen molecule.
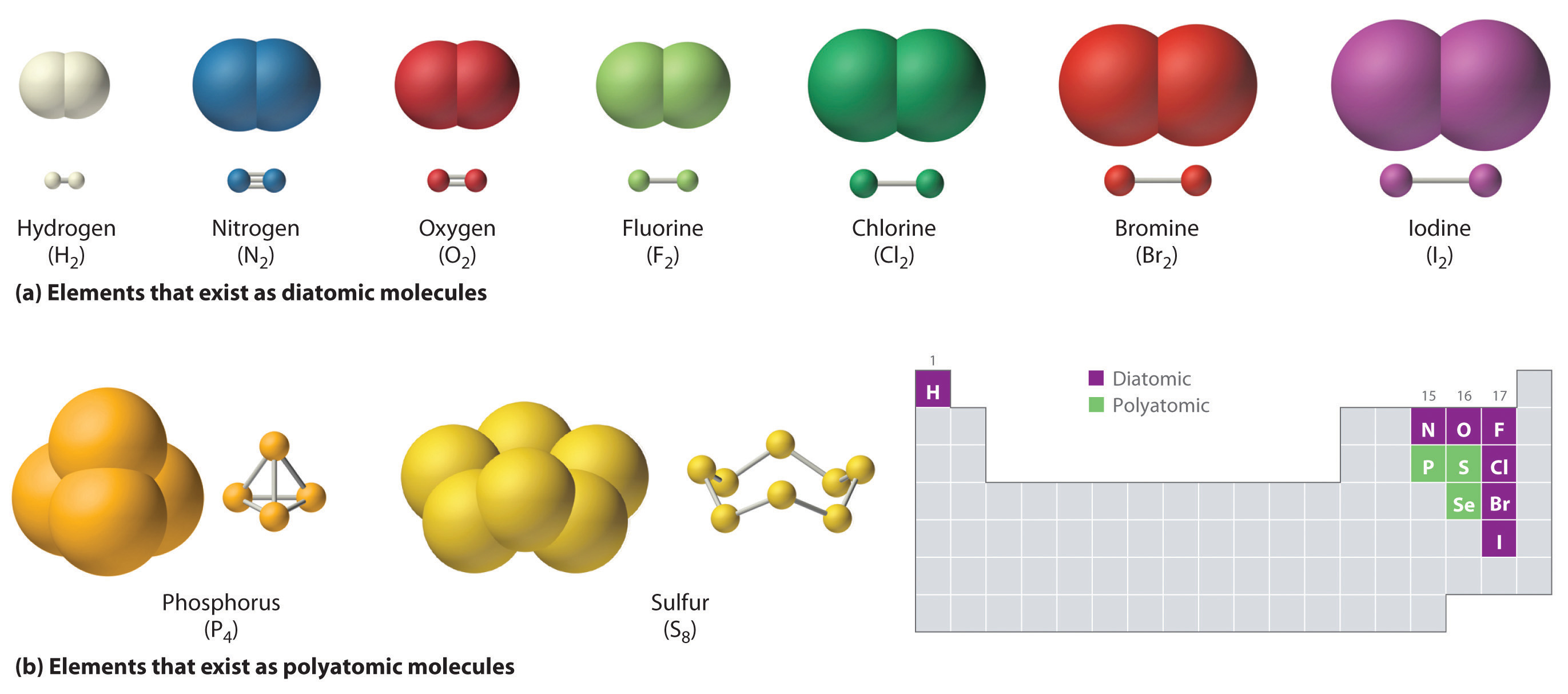
Each atom contributes one electron to the shared pair, helping both atoms achieve an octet in their valence shell. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. How does a covalent bond form? The simplest covalent bond exists in the diatomic hydrogen molecule. Living things are made up of atoms, but in most cases, those atoms aren’t just floating around individually. Various methods of showing a covalent bond. Two different atoms can also share electrons and form covalent bonds. The electrons involved are in the outer shells of the atoms. Web covalent bonds form between atoms with relatively high electron affinity and they form individual, separate molecules (figure below). It is fundamental to know the bonding characteristics of atoms.
covalent bond Definition, Properties, Examples, & Facts Britannica
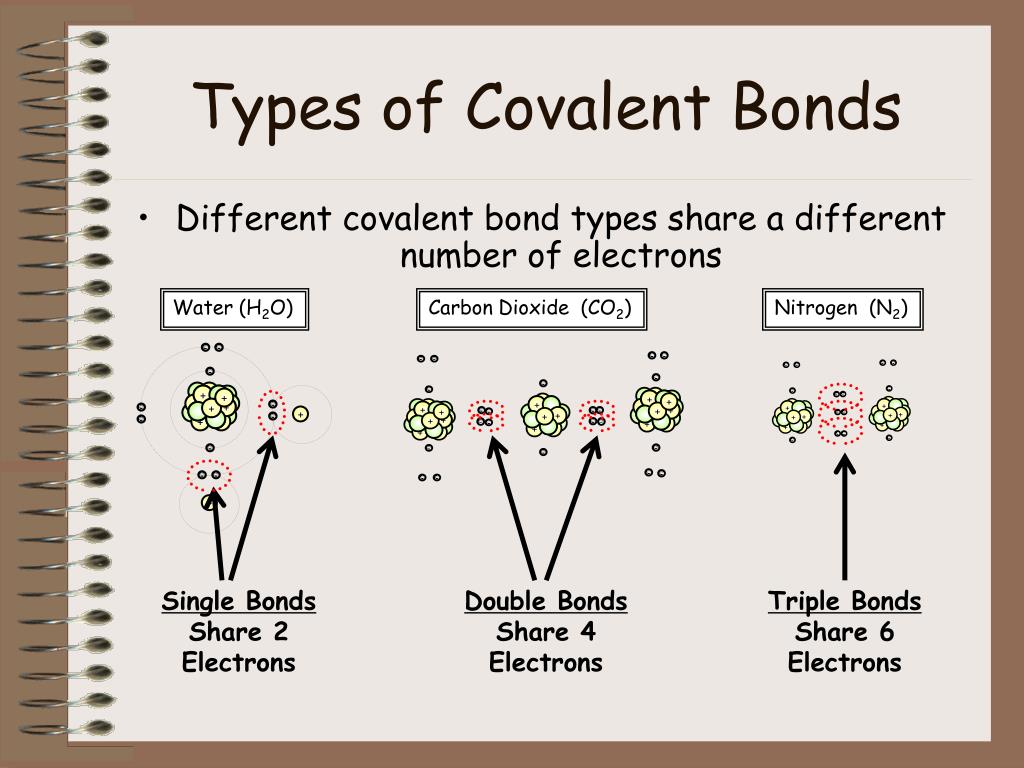
An example of a covalent compound is ammonia. A covalent bond is the force of attraction that holds together two nonmetal atoms that share a pair of electrons. What types of atoms tend to form the following types of. A triple bond is formed when three pairs of electrons are shared between the two participating atoms. For example, the hydrogen.
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download
When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent bond. Web covalent bonds involve the sharing of electron pairs between atoms. Do a covalent bond should necessarily have a difference in their electronegativities. Hydrogen bonds and london dispersion forces. Web.
Polar Covalent Bonds
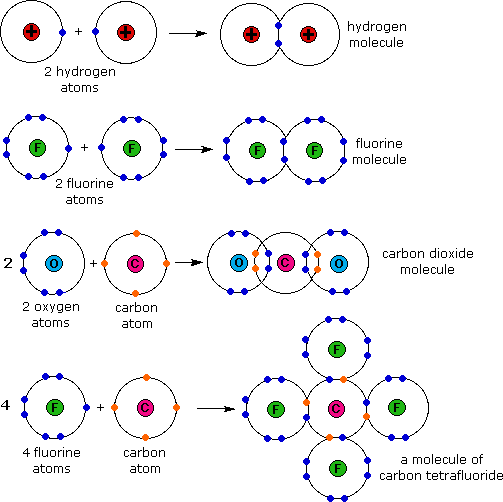
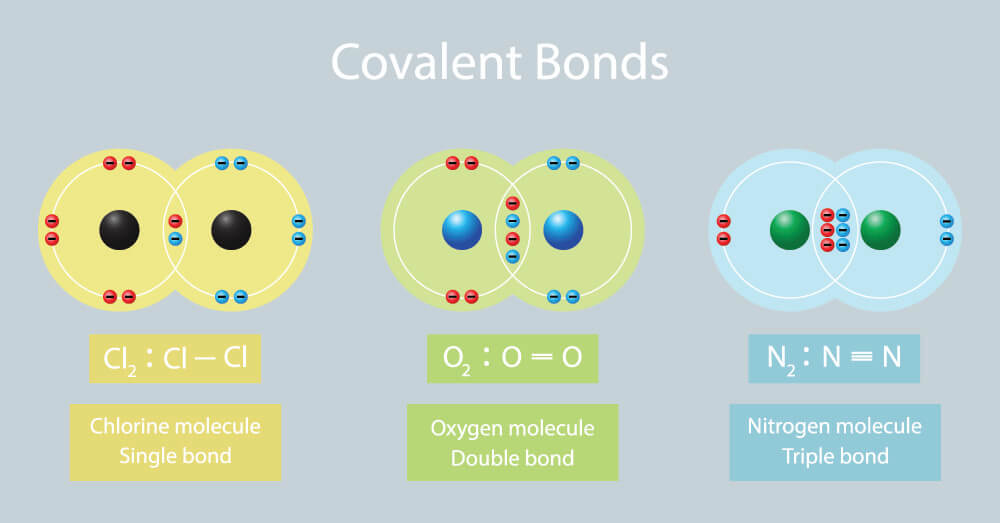
Web the two atoms can also share two pairs of electrons (a double bond) or three pairs of electrons (triple bond): Ions with opposite electrical charges attract d. Web introduction only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. Each h atom starts with a single electron.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Covalent bonds form between atoms of nonmetallic elements. Containing covalent bonds between two of the same type of atom are only a few examples of the vast number of molecules that can form. Web the shorter bond length has greater bond strength. In general, bonds are considered to be covalent if the electronegativity difference between the two atoms bonding is.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web what types of atoms form covalent bonds? For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. Based on the bond length, covalent bonds are of the following types. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). This type.
Covalent Bonds Biology for NonMajors I
Various methods of showing a covalent bond. Molecular bonds are another name for covalent bonds. Any object (such as a magnet, polar molecule or antenna), that is oppositely charged at two points (or poles). Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. Web nonmetal atoms frequently form covalent.
Covalent Bonding (Biology) — Definition & Role Expii
Web there are two main types of covalent bonds that can occur based on the electronegativity of the atoms involved: Web formation of covalent bonds. Living things are made up of atoms, but in most cases, those atoms aren’t just floating around individually. A type of chemical bond where two atoms are connected to each other by the sharing of.
EduMission Chemistry Form 4 Chapter 5 Covalent Bond
In covalent compounds, atoms form covalent bonds that consist of electron pairs shared between two adjacent atomic nuclei. Web a covalent bond is formed when electrons from both participating atoms are shared equally. Illustrates why this bond is formed. The pair of electrons involved in this type of bonding is known as a shared pair or bonding pair. Any object.
Covalent Enseñanza de química, Enlace químico, Enlace covalente
Each h atom starts with a single electron in its valence shell: Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). An example of a covalent compound is ammonia. Single covalent bonds between different atoms. Web molecular shape isomerism in organic compounds there are many types of.
Covalent Bond Biology Dictionary
An example of a covalent compound is ammonia. Web formation of covalent bonds. The sharing of bonding pairs will ensure that the atoms achieve stability in their outer shell, similar. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. In ionic bonding, atoms transfer electrons to each other.
Hydrogen Bonds And London Dispersion Forces.
In organic chemistry, when a molecule with a planar ring obeys hückel's rule, where the number of π electrons fit the formula 4 n + 2. The simplest covalent bond exists in the diatomic hydrogen molecule. Web introduction only when two atoms of the same element form a covalent bond are the shared electrons actually shared equally between the atoms. Web a covalent bond is formed when electrons from both participating atoms are shared equally.
For Example, The Hydrogen Molecule, H 2, Contains A Covalent Bond Between Its Two Hydrogen Atoms.
Figure 7.4 illustrates why this bond is formed. The two most basic types of bonds are characterized as either ionic or covalent. Definition, functions, types, and faqs jul 7, 2022 covalent bond electronic configuration has been a very important topic in chemistry over the years. Web by sania jakati in this, article we are going to study examples of various covalent bond types of atoms.
What Is A Covalent Bond?
Web double bonds triple bond. Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web as a general rule, covalent bonds are formed between elements lying toward the right in the periodic table (i.e., the nonmetals). Web chemistry biology robotics coding covalent bond :
Web Covalent Bonds Form Between Atoms With Relatively High Electron Affinity And They Form Individual, Separate Molecules (Figure Below).
A type of chemical bond where two atoms are connected to each other by the sharing of two or more electrons. When atoms of different elements share electrons through covalent bonding, the electron will be drawn more toward the atom with the higher e lectronegativity resulting in a polar covalent bond. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. A triple bond is formed when three pairs of electrons are shared between the two participating atoms.