What Types Of Atoms Typically Form Covalent Bonds
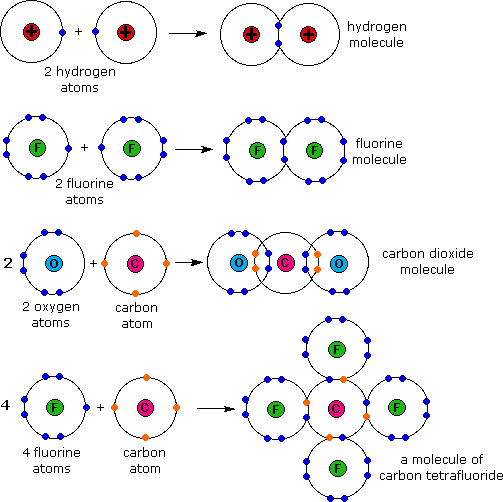
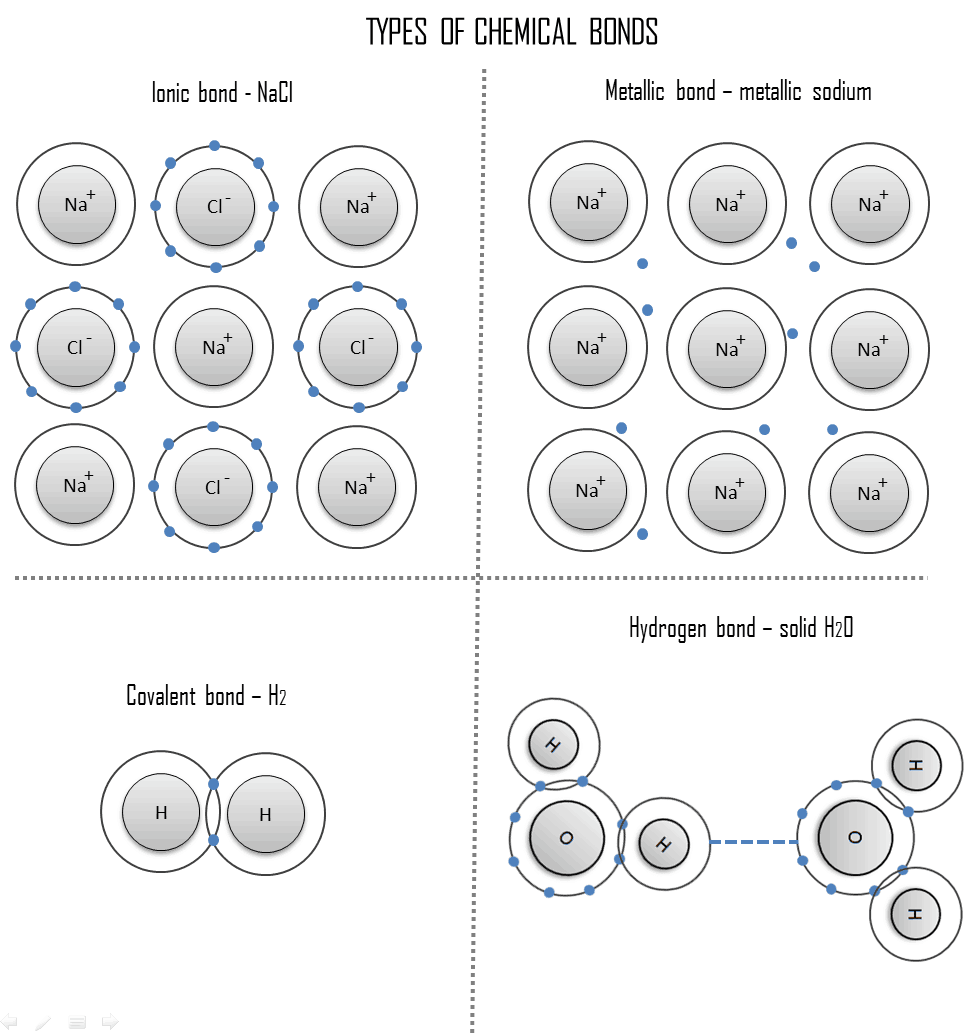
What Types Of Atoms Typically Form Covalent Bonds - Web octet rule exceptions. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Web main types of chemical bonds. Web molecules most covalently bonded substances consist of small molecules. In general, bonds are considered to be covalent if the electronegativity difference between the two. A covalent bond is usually formed between the atoms that belong to. Covalent bonding generally happens between nonmetals. An ionic bond is formed when. Web this is a covalent bond, a bond in which atoms share electrons. For example, the hydrogen molecule, h 2,.
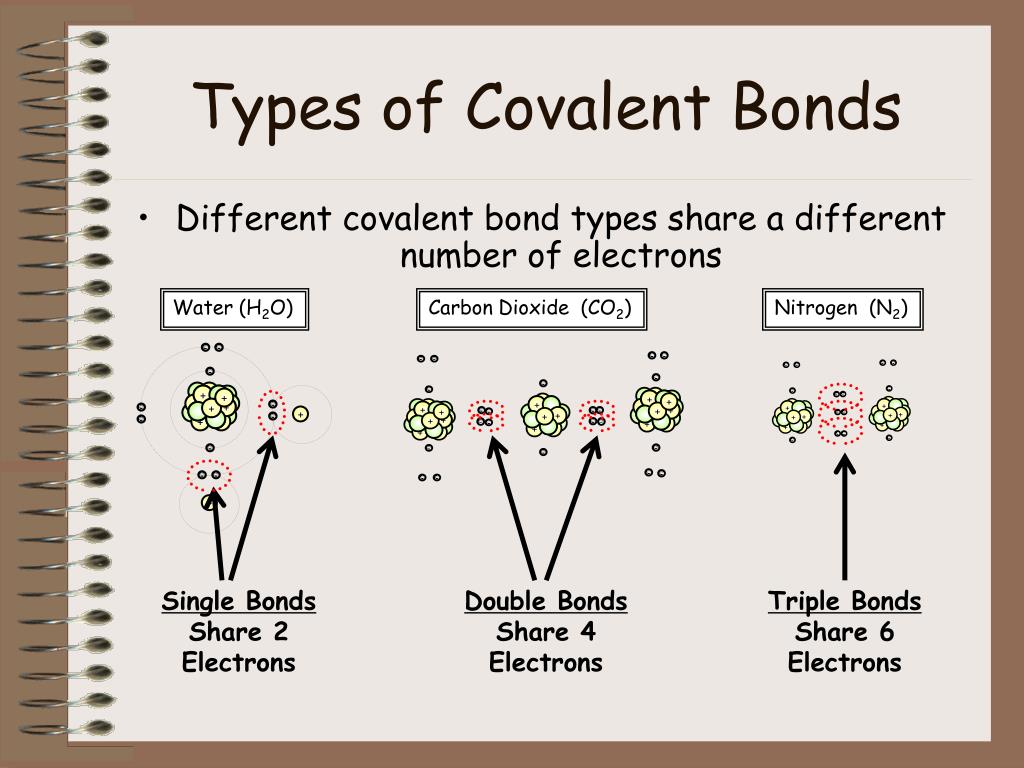
Web iit jee study material covalent bond covalent bond a covalent bond is formed by the equal sharing of electrons from both participating atoms. Web non metals form covalent bonds in order to achieve a stable electron configuration similar to that of the noble gases. Web the two atoms can also share two pairs of electrons (a double bond) or three pairs of electrons (triple bond): A covalent bond is usually formed between the atoms that belong to. Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. Add extra if the species has negative charges and remove some for every positive charge on the. Web the best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. Web molecules most covalently bonded substances consist of small molecules. Web this is a covalent bond, a bond in which atoms share electrons. Is energy always released when.
Web iit jee study material covalent bond covalent bond a covalent bond is formed by the equal sharing of electrons from both participating atoms. Web molecules most covalently bonded substances consist of small molecules. Web this is a covalent bond, a bond in which atoms share electrons. Covalent bonding is the type of bond that holds. In lewis theory, a pair of electrons, known as a bonding pair, is. Add extra if the species has negative charges and remove some for every positive charge on the. Web the octet rule can be satisfied by the sharing of electrons between atoms to form covalent bonds. Web covalent bonds form between atoms with relatively high electron affinity and they form individual, separate molecules (figure below). These bonds are stronger and much more common than are ionic bonds in the. An ionic bond is formed when.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Covalent bonding generally happens between nonmetals. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Count the total number of valence electrons. Web by sania jakati in this, article we are going to study examples of various covalent bond types of atoms. These bonds are stronger and much more common than are ionic bonds.
Covalent Bond Biology Dictionary
Various methods of showing a covalent. These bonds are stronger and much more common than are ionic bonds in the. Web octet rule exceptions. Web the two atoms can also share two pairs of electrons (a double bond) or three pairs of electrons (triple bond): Add extra if the species has negative charges and remove some for every positive charge.
PPT Notes 53 Covalent Bonds PowerPoint Presentation, free download
Web octet rule exceptions. Web the best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. Web covalent bonds form between atoms with relatively high electron affinity and they form individual, separate molecules (figure below). Web non metals form covalent bonds in order.
11 Types of scientific changes with examples
Web non metals form covalent bonds in order to achieve a stable electron configuration similar to that of the noble gases. An ionic bond is formed when. For example, the hydrogen molecule, h 2,. Web the two atoms can also share two pairs of electrons (a double bond) or three pairs of electrons (triple bond): Web this is a covalent.
Types of covalent bonds YouTube
An ionic bond is formed when. Web covalent bonds form between atoms with relatively high electron affinity and they form individual, separate molecules (figure below). Web main types of chemical bonds. Covalent bonding is the type of bond that holds. Various methods of showing a covalent.
Forms of Binding in Crystals Overall Science
Web the best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. Web this is a covalent bond, a bond in which atoms share electrons. Covalent bonding is the type of bond that holds. An ionic bond is formed when. A molecule is.
Covalent Bonds Biology for NonMajors I
The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Web this is a covalent bond, a bond in which atoms share electrons. Various methods of showing a covalent. Web the best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
In lewis theory, a pair of electrons, known as a bonding pair, is. Web main types of chemical bonds. These bonds are stronger and much more common than are ionic bonds in the. Web octet rule exceptions. Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond.
The Periodic Table and Bonding Mrs. Sanborn's Site
In lewis theory, a pair of electrons, known as a bonding pair, is. Add extra if the species has negative charges and remove some for every positive charge on the. For example, the hydrogen molecule, h 2,. Web covalent bonds form between atoms with relatively high electron affinity and they form individual, separate molecules (figure below). These bonds are stronger.
Covalent Bond vs Metallic Bond Definition Material Properties
Web covalent bonds form between atoms of nonmetallic elements. Web the two atoms can also share two pairs of electrons (a double bond) or three pairs of electrons (triple bond): An ionic bond is formed when. Covalent bonding generally happens between nonmetals. Web octet rule exceptions.
Count The Total Number Of Valence Electrons.
Is energy always released when. Web main types of chemical bonds. The two main types of bonds formed between atoms are ionic bonds and covalent bonds. Web there are two main types of covalent bonds that can occur based on the electronegativity of the atoms involved:
Web Non Metals Form Covalent Bonds In Order To Achieve A Stable Electron Configuration Similar To That Of The Noble Gases.
Web iit jee study material covalent bond covalent bond a covalent bond is formed by the equal sharing of electrons from both participating atoms. Web the two atoms can also share two pairs of electrons (a double bond) or three pairs of electrons (triple bond): Covalent bonding is the type of bond that holds. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms.
Web Molecules Most Covalently Bonded Substances Consist Of Small Molecules.
Various methods of showing a covalent. A covalent bond is usually formed between the atoms that belong to. Web octet rule exceptions. Add extra if the species has negative charges and remove some for every positive charge on the.
Web The Sharing Of Electrons Between Atoms Is Called A Covalent Bond, And The Two Electrons That Join Atoms In A Covalent Bond Are Called A Bonding Pair Of Electrons.
Web covalent bonds form between atoms with relatively high electron affinity and they form individual, separate molecules (figure below). Covalent bonding generally happens between nonmetals. These bonds are stronger and much more common than are ionic bonds in the. Web by sania jakati in this, article we are going to study examples of various covalent bond types of atoms.