What Types Of Elements Form Ionic Compounds
What Types Of Elements Form Ionic Compounds - Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Web video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. Web an ionic compound is made of ions, charged particles formed when an atom or group of atoms acquire or lose electrons. For example, cabr 2 contains a metallic element (calcium, a group 2 [or 2a]. Compounds that do not contain ions, but instead consist of. Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table. Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Web compounds formed from positive and negative ions are called ionic compounds. Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound.
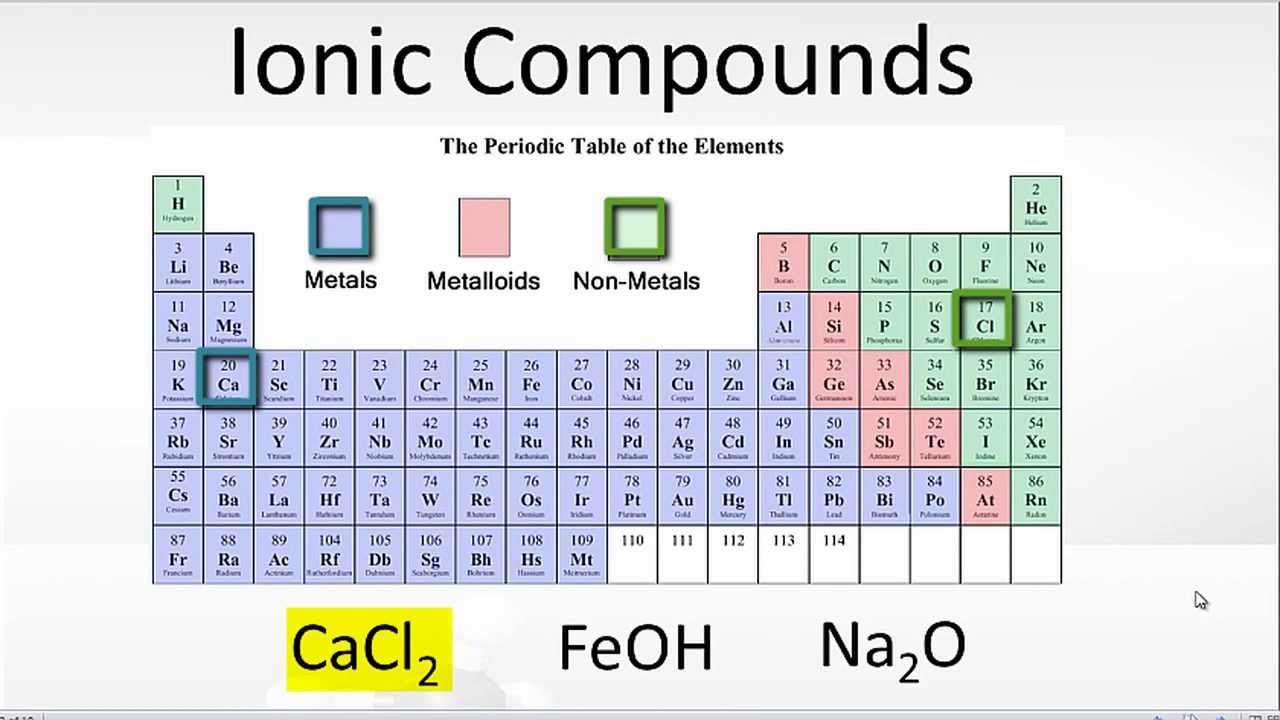
Ionic compounds form by two ways: Then, identify the anion and write down its symbol and. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table. Web compounds that contain ions are called ionic compounds. Compounds between metal and nonmetal elements are usually ionic. Ionic compounds form as electrons move from metal atoms to. The two most basic types of. Web ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,.
Web video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. Ions form when atoms lose or gain electrons to obtain a full outer shell:. Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. Web compounds that contain ions are called ionic compounds. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Ionic compounds form by two ways: Web ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist. Compounds between metal and nonmetal elements are usually ionic. Web ionic compounds are pure substances consisting of chemically bonded ions.
Ionic Bond Definition, Types, Properties & Examples
Compounds that do not contain ions, but instead consist of. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Compounds between metal and nonmetal elements are usually ionic. Web ionic compounds generally form from metals and nonmetals. Ions form when atoms lose or gain electrons to obtain a full outer shell:.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Ionic compounds form by two ways: The two most basic types of. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Web an ionic compound is made of ions, charged particles formed when an atom or group of atoms acquire or lose electrons. To find the formula of an ionic compound, first identify.
Naming Simple Ionic Compounds Pathways to Chemistry
Web compounds that contain ions are called ionic compounds. Ionic compounds generally form from metals and nonmetals. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Compounds that do not contain ions, but instead consist of. Web ionic compounds consist of positively and negatively charged ions held.
Examples of Ionic Bonds and Compounds
Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. The two most basic types of. Compounds that do not contain ions, but instead consist of. Web video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. Web when atoms of.
Periodic Table Ions List Periodic Table Timeline
To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The two most basic types of. Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. Compounds that do not contain ions, but instead consist of. Compounds that do not contain ions, but.
Ionic Bond Definition, Types, Properties & Examples
Ionic compounds form by two ways: Ionic compounds generally form from metals and nonmetals. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Web compounds that contain ions are called ionic compounds. When they do, they become monatomicions.
Ionic Bond Definition, Types, Properties & Examples
Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Web an ionic compound is made of ions, charged particles formed when an atom or group of atoms acquire or lose electrons. Web ionic compounds generally form from.
2.7 Ions and Ionic Compounds Chemistry LibreTexts
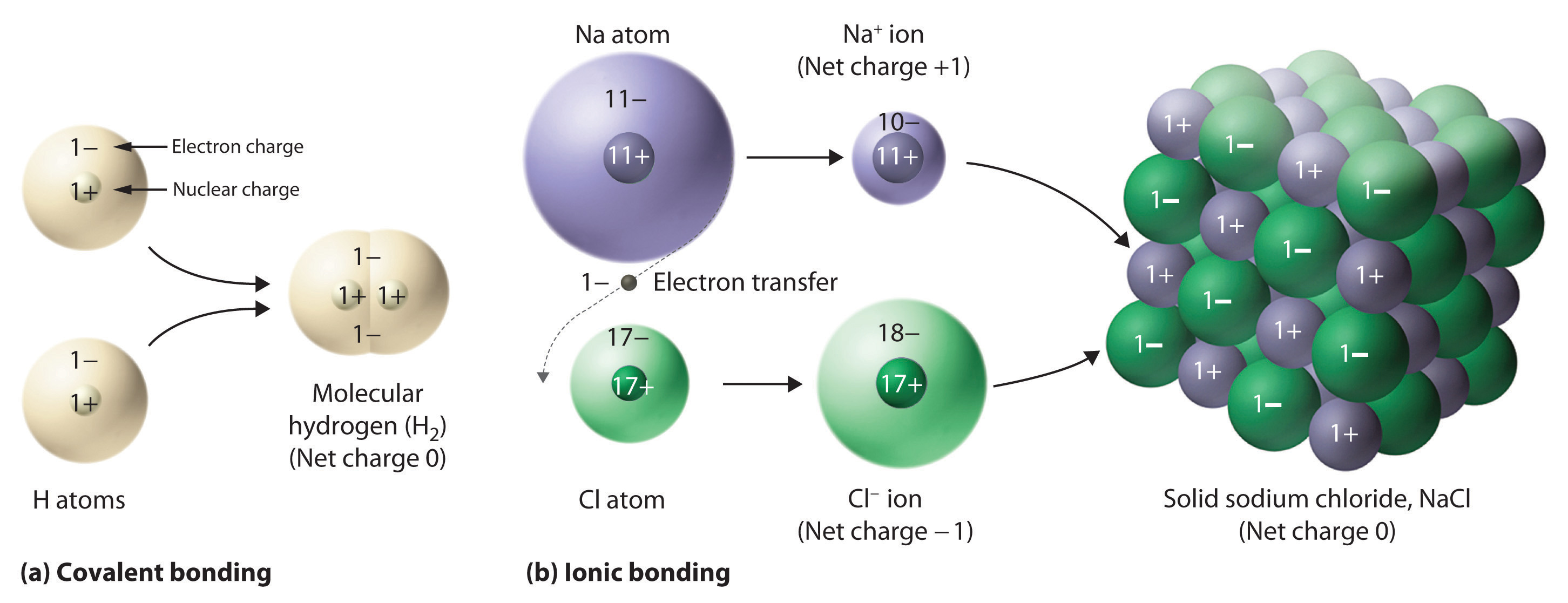
Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Then, identify the anion and write down its symbol and. Web ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist. Ionic compounds form as electrons move from.
Ionic bonding Wikipedia
Ionic compounds form by two ways: Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web compounds that contain ions are called ionic compounds. Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. When they do, they become monatomicions. Ionic compounds form by two ways: Ions form when atoms lose or gain electrons to obtain a full outer shell:.
Web Compounds Formed From Positive And Negative Ions Are Called Ionic Compounds.
Web ionic compounds generally form from metals and nonmetals. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Individual atoms can gain or lose electrons. Web video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge.
Web Ionic Compounds Are Pure Substances Consisting Of Chemically Bonded Ions.
Web glossary learning objectives define ionic compounds predict the type of compound formed from elements based on their location within the periodic table. Compounds between metal and nonmetal elements are usually ionic. Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. When they do, they become monatomicions.
Compounds That Do Not Contain Ions, But Instead Consist Of.
Web an ionic compound is made of ions, charged particles formed when an atom or group of atoms acquire or lose electrons. Compounds that do not contain ions, but instead consist of atoms bonded tightly together in molecules. Web ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist. Ionic compounds generally form from metals and nonmetals.
Ionic Compounds Form By Two Ways:
Web when atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic. Web the elements that tend to form ionic compounds include cadmium, chromium, cobalt, iron, gold, copper, nickel, manganese, mercury, silver, zinc, tin,. Ionic compounds form as electrons move from metal atoms to. Ions form when atoms lose or gain electrons to obtain a full outer shell:.



/ionic-bond-58fd4ea73df78ca1590682ad.jpg)



.PNG)