Which Elements Form Negative Ions
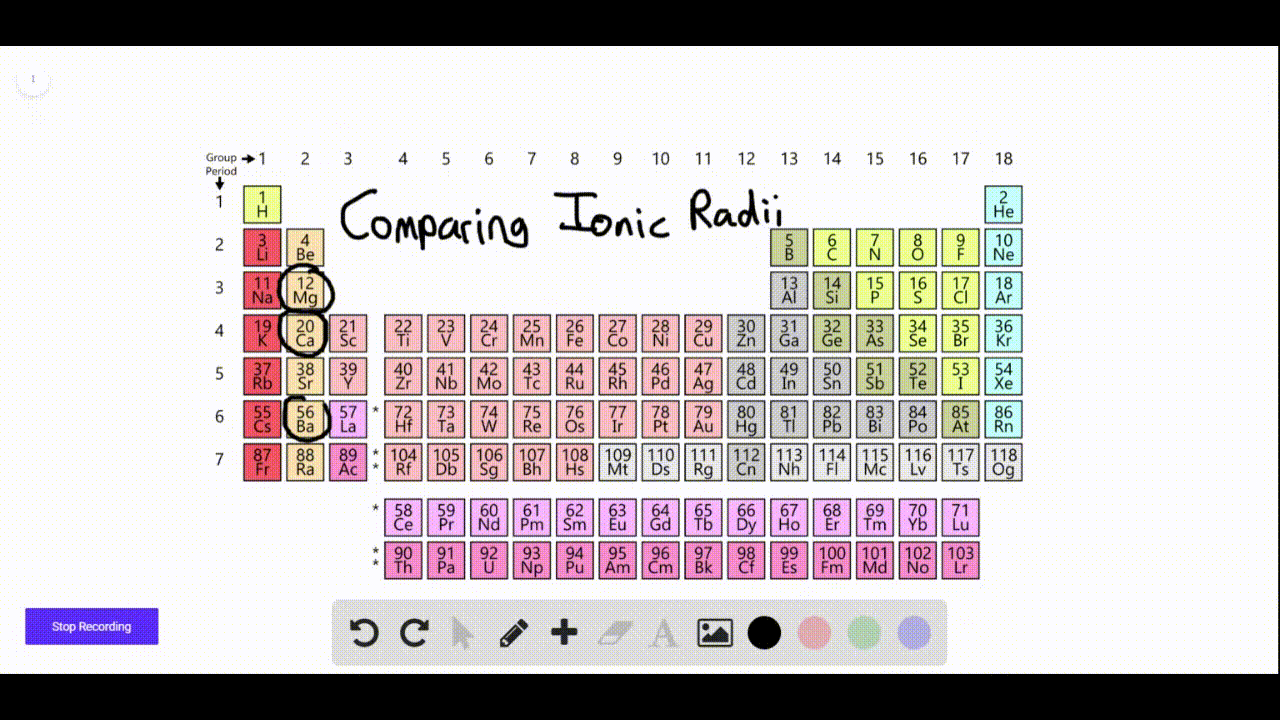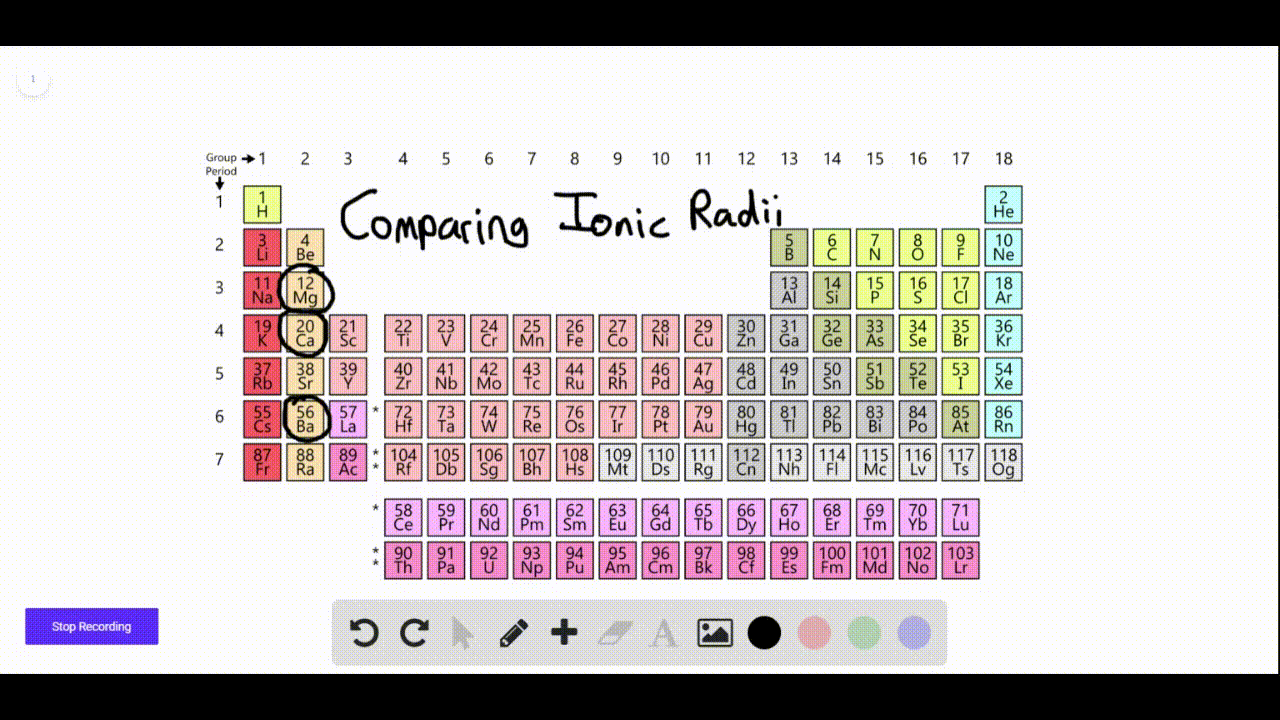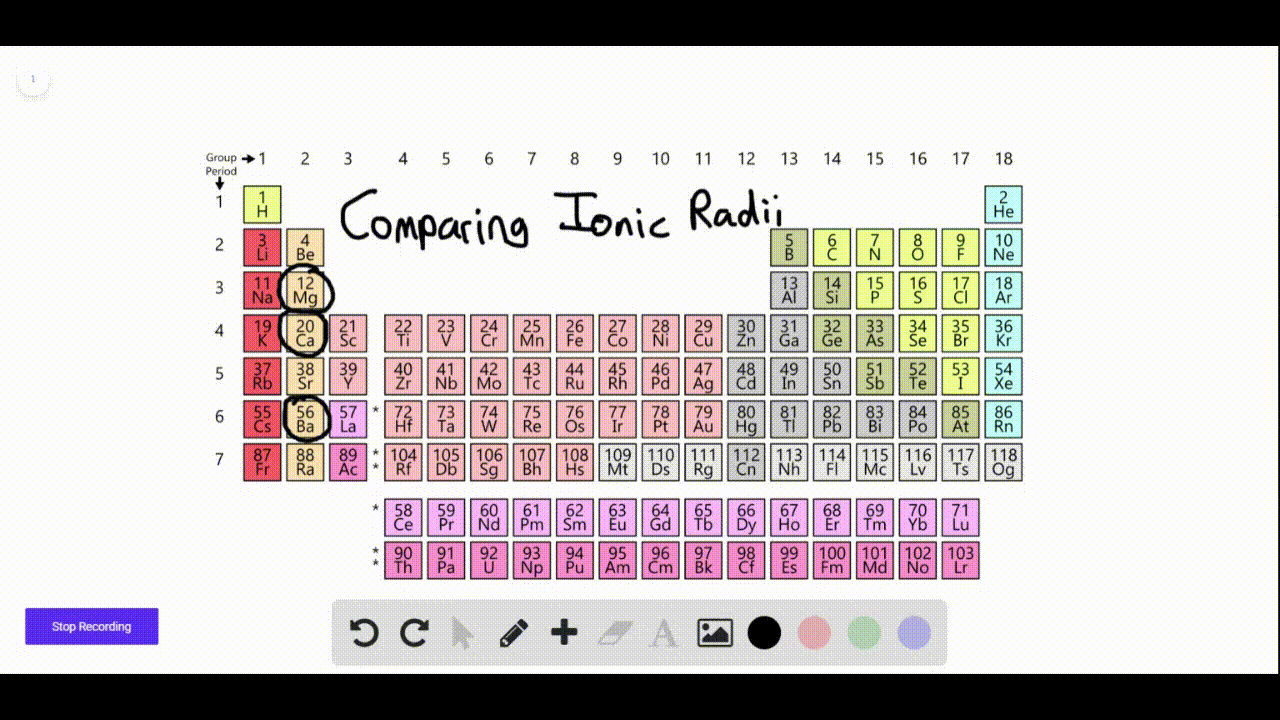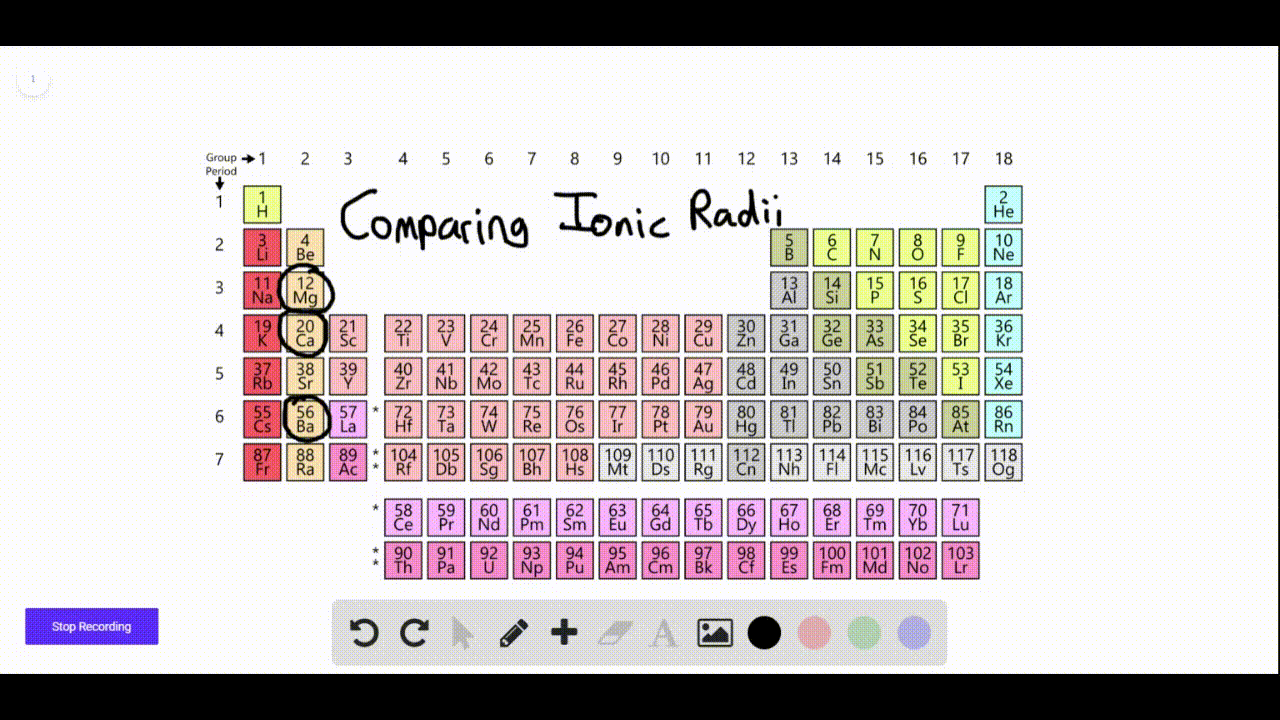
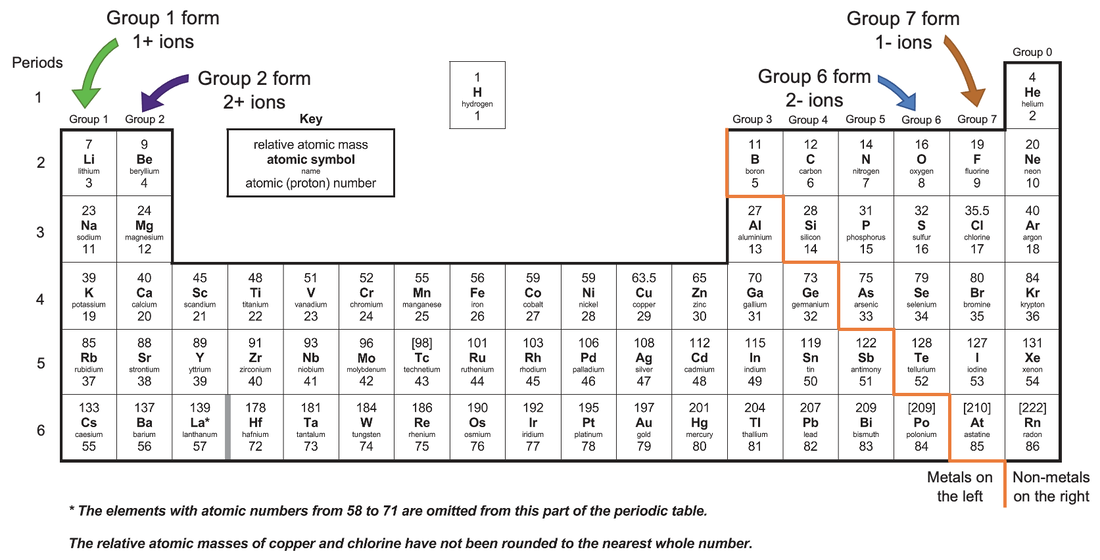
Which Elements Form Negative Ions - Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations; Electrons have a negative charge, whereas. Oxygen is in group 6. The protons do not change. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. Web anions are the negative ions formed from the gain of one or more electrons. Web it is going to have six electrons and that's what makes it neutral. Web forming negative ions (anions) atoms gain electrons in their outer shell when they form. For a cation, simply use the name of the element and add the word ion (or if you want to be more specific, add cation) after the.
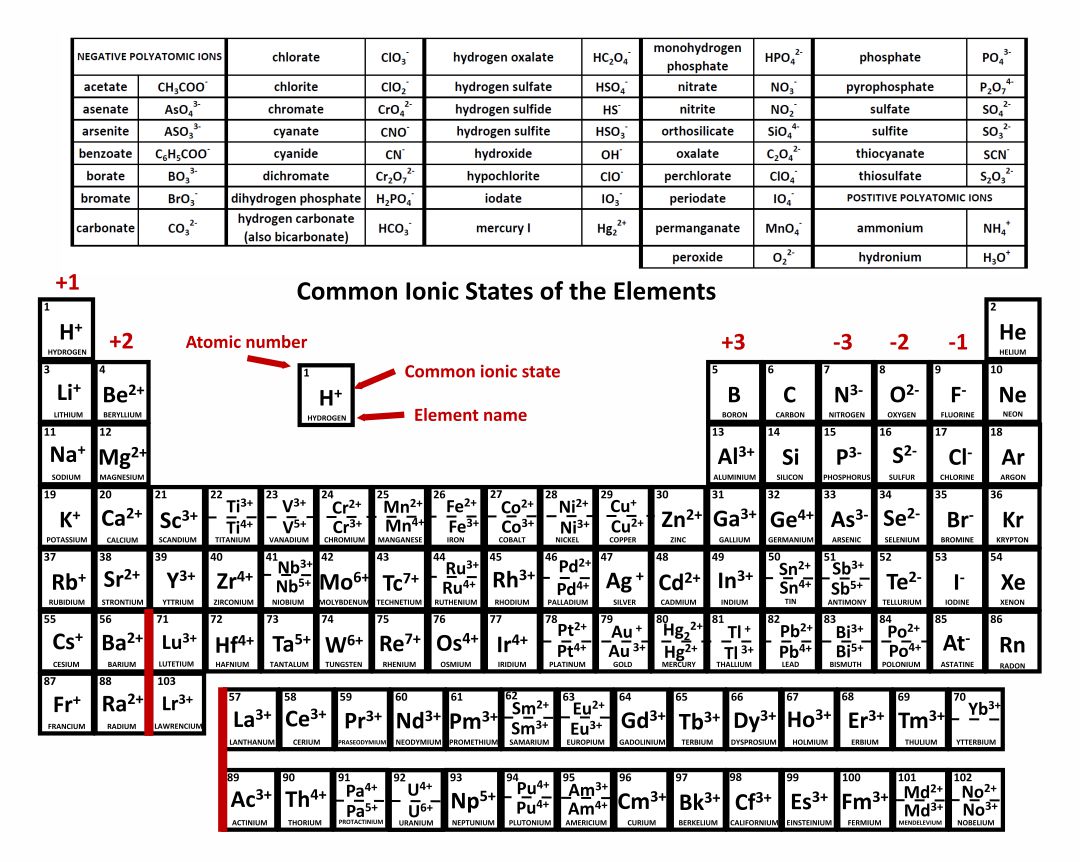
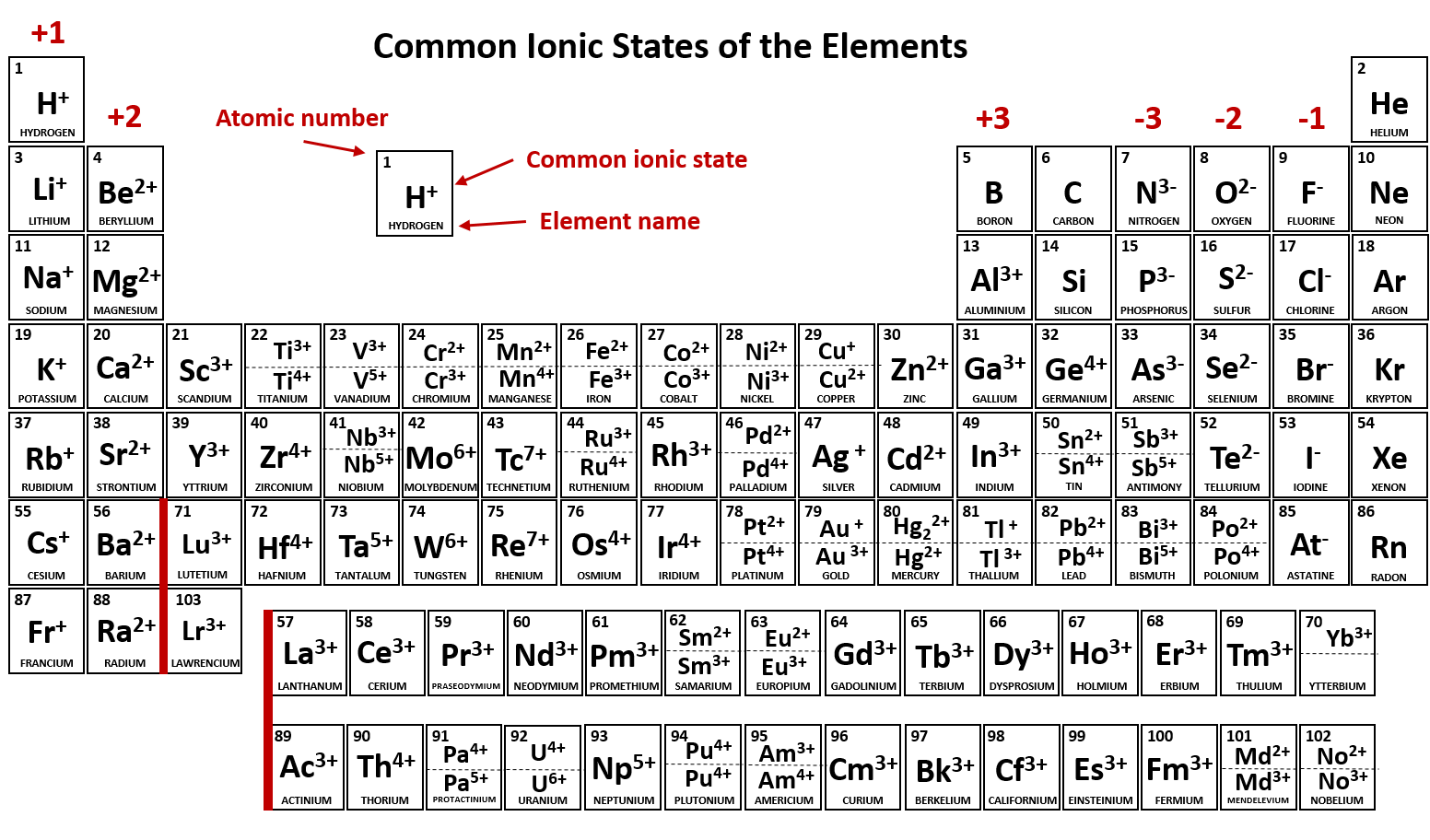
Naming an ion is straightforward. Negative ions are called anions. Electrons have a negative charge, whereas. Oxygen is in group 6. Most atoms do not have eight electrons in their valence electron. Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. Because of this configuration, they will. Web a negative ion is formed by the addition of negatively charged electrons. Web an ion is defined as an atom or group of atoms where the number of electrons is not equal to the number of protons. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges.
Web it is going to have six electrons and that's what makes it neutral. Web anions are the negative ions formed from the gain of one or more electrons. Web an ion is defined as an atom or group of atoms where the number of electrons is not equal to the number of protons. Produce negative ions, such as oxygen. Web ions are formed as a result of this addition or removal of electrons from the atom. When nonmetal atoms gain electrons, they often do so until their outermost principal. Oxygen is in group 6. Ionic compounds form as electrons move from metal atoms to. The right hand side of the periodic table as we face it. You have the six positive charges and the six negative charges.
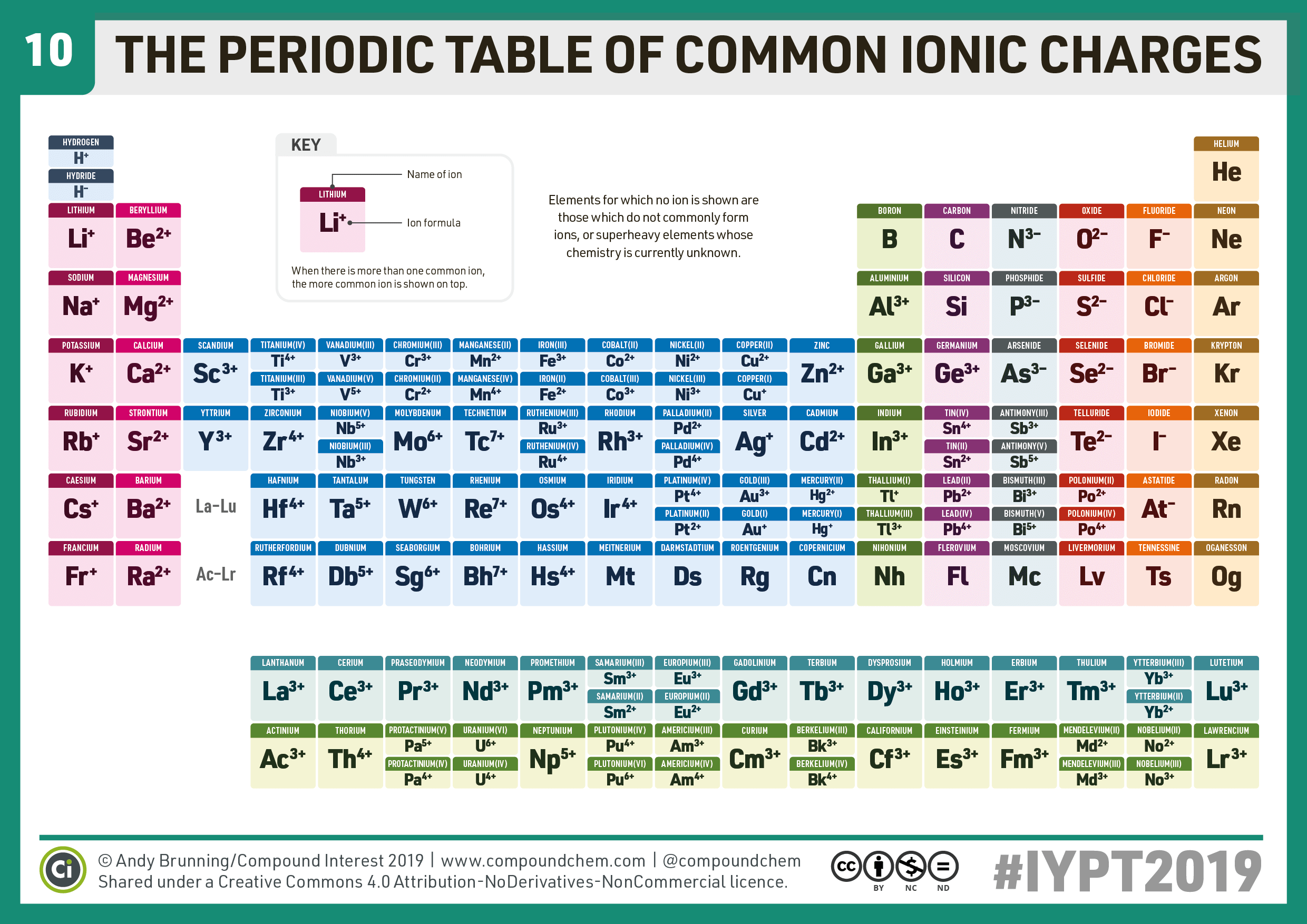
10 Periodic Table of Common Ions Compound Interest
Web an ion is defined as an atom or group of atoms where the number of electrons is not equal to the number of protons. Now you could have a carbon. These are the elements with 7 valence electrons. Web ions are formed as a result of this addition or removal of electrons from the atom. Produce negative ions, such.
SOLVEDAn element forms a negative ion when ionized. On what side of
Most atoms do not have eight electrons in their valence electron. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Oxygen is in group 6. Web learning objectives define the two types of ions. The ions formed are negative, because they have more electrons than protons
C2 B) Ions from the Periodic Table AQA Combined Science Trilogy Elevise
Use lewis diagrams to illustrate ion formation. Now you could have a carbon. Electrons have a negative charge, whereas. The right hand side of the periodic table as we face it. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges.
10 Best Printable Periodic Table Of Ions
Now you could have a carbon. Positively charged ions are called cations; Produce negative ions, such as oxygen. When nonmetal atoms gain electrons, they often do so until their outermost principal. Ionic compounds form as electrons move from metal atoms to.
3.2 Ions The Basics of General, Organic, and Biological Chemistry
The protons do not change. Oxygen is in group 6. The parent atoms are oxygenating species: Web a negative ion is formed by the addition of negatively charged electrons. Because of this configuration, they will.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
These are the elements with 7 valence electrons. Ionic compounds form as electrons move from metal atoms to. Produce negative ions, such as oxygen. Web the elements that would form negative ions the easiest are the halogens. Electrons have a negative charge, whereas.
The Chemistry of Ion Exchange WCP Online
Web the elements that would form negative ions the easiest are the halogens. Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. These are the elements with 7 valence electrons. The parent atoms are oxygenating species: Because of this configuration, they will.
Periodic Table Ionic Charges Pdf Awesome Home
The protons do not change. Use lewis diagrams to illustrate ion formation. Now you could have a carbon. Web forming negative ions (anions) atoms gain electrons in their outer shell when they form. Negative ions, known as anions, form when an atom gains electrons and now has more electrons.
Ions
The ions formed are negative, because they have more electrons than protons Web learning objectives define the two types of ions. Web 5 rows forming negative ions. Negative ions are called anions. Negative ions, known as anions, form when an atom gains electrons and now has more electrons.
Electron Affinity of The Elements
Web an ionic bond is an electrostatic attraction between the positive and negative ions of a chemical compound. You have the six positive charges and the six negative charges. Web 5 rows forming negative ions. For a cation, simply use the name of the element and add the word ion (or if you want to be more specific, add cation).
Web Learning Objectives Define The Two Types Of Ions.
Web moving from the far right to the left on the periodic table, elements often form anions with a negative charge equal to the number of groups moved left from the noble gases. Electrons have a negative charge, whereas. For a cation, simply use the name of the element and add the word ion (or if you want to be more specific, add cation) after the. Web the elements that would form negative ions the easiest are the halogens.
Negative Ions Are Called Anions.
Oxygen is in group 6. The right hand side of the periodic table as we face it. Web anions are the negative ions formed from the gain of one or more electrons. Positively charged ions are called cations;
The Parent Atoms Are Oxygenating Species:
Use lewis diagrams to illustrate ion formation. Web in this way, nitrogen, oxygen, fluorine, chlorine, etc., are formed. Web a negative ion is formed by the addition of negatively charged electrons. When nonmetal atoms gain electrons, they often do so until their outermost principal.
Web An Ionic Bond Is An Electrostatic Attraction Between The Positive And Negative Ions Of A Chemical Compound.
The protons do not change. Naming an ion is straightforward. Most atoms do not have eight electrons in their valence electron. Web an ion is defined as an atom or group of atoms where the number of electrons is not equal to the number of protons.