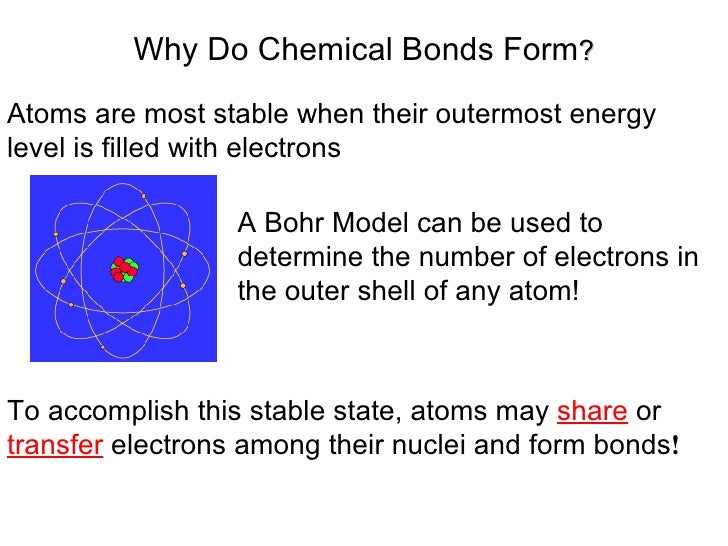
Why Do Elements Form Bonds
Why Do Elements Form Bonds - Web 1 what are chemical bonds and why do they form? It might be stable there,. Web answer (1 of 4): The matter in a system will always trend towards the state where there is the lowest potential energy. An atom that shares one or more of its. That chemical bonding involves atoms being held together by. Posted by city chemical on monday, 01 april 2019 attraction between atoms or ions leads to a. Web how do elements join to from compounds? Chemical bonds certainly contain potential energy, and the atoms want to move to a lower potential energy (become more stable). Web generally, the greater the risk, the higher the interest paid by a bond.
Web generally, the greater the risk, the higher the interest paid by a bond. Molecules are composed of atoms that have bonded and released the extra energy with them as bond formation energy.this bond can either be. Web how do elements join to from compounds? Think of a ball at the top of a hill. Returns on bonds are usually lower than those of stocks, but the. These bonds can be ionic or covalent. Elements share electrons to form. Chemical bonds certainly contain potential energy, and the atoms want to move to a lower potential energy (become more stable). Web because the addition of atoms to the π bond of alkenes to form new σ bonds is a general and characteristic reaction of alkenes, alkenes are said to be unsaturated. It might be stable there,.
Think of a ball at the top of a hill. Returns on bonds are usually lower than those of stocks, but the. These bonds can be ionic or covalent. Web atoms form two basic bonds, covalent or ionic bonds, to fill the full outer shell of electrons. Protons and neutrons compose the positively charged nucleus whilst electrons orbit in orbitals (or. Because positive and negative charges attract, these ions stay together and form an ionic bond, or a bond. Web 1 what are chemical bonds and why do they form? Web answer (1 of 4): Web generally, the greater the risk, the higher the interest paid by a bond. The matter in a system will always trend towards the state where there is the lowest potential energy.
Chemical Bonds Boundless Anatomy and Physiology
Web how do elements join to from compounds? Web answer (1 of 4): Elements are made of protons, neutrons and electrons. Web when atoms form bonds, they can achieve a stable electron arrangement. The matter in a system will always trend towards the state where there is the lowest potential energy.
Why Do Most Atoms Form Chemical Bonds? Sciencing
That atoms must collide (bump together) in order to form chemical bonds. Web how do elements join to from compounds? Posted by city chemical on monday, 01 april 2019 attraction between atoms or ions leads to a. Web 1 what are chemical bonds and why do they form? Chemical bonds certainly contain potential energy, and the atoms want to move.
CH105 Chapter 3 Ionic and Covelent Bonding Chemistry
Web the element accepting the electron is now negatively charged. These bonds can be ionic or covalent. The electrons involved are in the outer shells of the atoms. Web when atoms form bonds, they can achieve a stable electron arrangement. Think of a ball at the top of a hill.
Why do atoms form bonds? Video] O Level Secondary Chemistry
Elements share electrons to form. Web because the addition of atoms to the π bond of alkenes to form new σ bonds is a general and characteristic reaction of alkenes, alkenes are said to be unsaturated. Web the element accepting the electron is now negatively charged. Web answer (1 of 4): Think of a ball at the top of a.
Biochemistry Honors
The electrons involved are in the outer shells of the atoms. Web the element accepting the electron is now negatively charged. Web generally, the greater the risk, the higher the interest paid by a bond. Web when atoms form bonds, they can achieve a stable electron arrangement. It might be stable there,.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Molecules are composed of atoms that have bonded and released the extra energy with them as bond formation energy.this bond can either be. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Think of a ball at the top of a hill. These bonds can be ionic.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Web because the addition of atoms to the π bond of alkenes to form new σ bonds is a general and characteristic reaction of alkenes, alkenes are said to be unsaturated. Because positive and negative charges attract, these ions stay together and form an ionic bond, or a bond. Elements share electrons to form. Web answer (1 of 16): That.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Returns on bonds are usually lower than those of stocks, but the. That chemical bonding involves atoms being held together by. Think of a ball at the top of a hill. Elements share electrons to form. The electrons involved are in the outer shells of the atoms.
Forms of Binding in Crystals Overall Science
Web 1 what are chemical bonds and why do they form? An atom that shares one or more of its. These bonds can be ionic or covalent. Elements are made of protons, neutrons and electrons. Web answer (1 of 16):
Elements and Chemical Bonds
Molecules are composed of atoms that have bonded and released the extra energy with them as bond formation energy.this bond can either be. Web when atoms form bonds, they can achieve a stable electron arrangement. Web because the addition of atoms to the π bond of alkenes to form new σ bonds is a general and characteristic reaction of alkenes,.
The Electrons Involved Are In The Outer Shells Of The Atoms.
The matter in a system will always trend towards the state where there is the lowest potential energy. Web generally, the greater the risk, the higher the interest paid by a bond. These bonds can be ionic or covalent. Web how do elements join to from compounds?
Chemical Bonds Certainly Contain Potential Energy, And The Atoms Want To Move To A Lower Potential Energy (Become More Stable).
When methane, ch4, forms, the. Think of a ball at the top of a hill. An atom that shares one or more of its. Web answer (1 of 4):
It Might Be Stable There,.
Elements are made of protons, neutrons and electrons. The bond may result from the electrostatic. Protons and neutrons compose the positively charged nucleus whilst electrons orbit in orbitals (or. Web 1 what are chemical bonds and why do they form?
Web The Element Accepting The Electron Is Now Negatively Charged.
Web answer (1 of 16): That chemical bonding involves atoms being held together by. Web because the addition of atoms to the π bond of alkenes to form new σ bonds is a general and characteristic reaction of alkenes, alkenes are said to be unsaturated. Posted by city chemical on monday, 01 april 2019 attraction between atoms or ions leads to a.


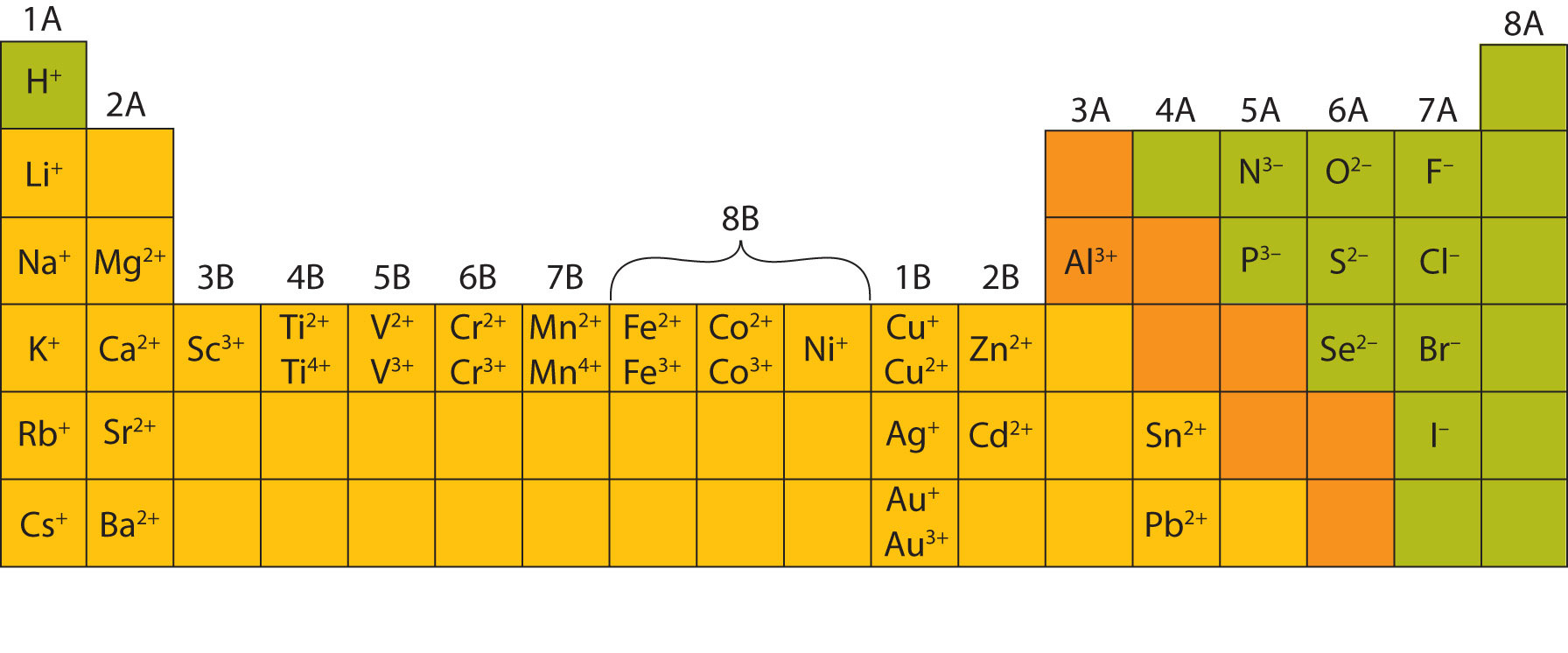
![Why do atoms form bonds? Video] O Level Secondary Chemistry](https://icandochemistry942105908.files.wordpress.com/2021/06/thumb-1.png?w=1200)